Organs Made to Order
It won’t be long before surgeons routinely install replacement body parts created in the laboratory
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Organs-Order-ear-631.jpg)
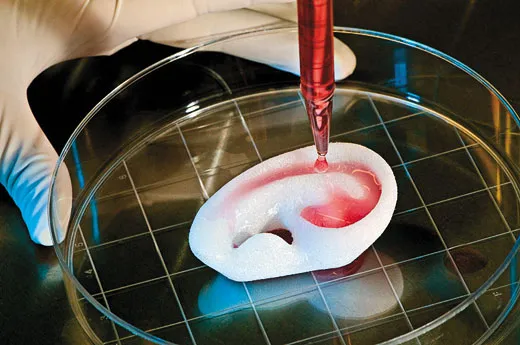
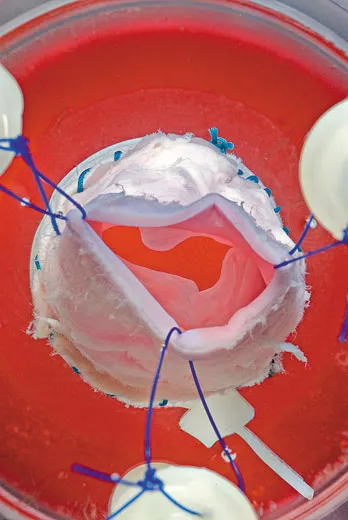
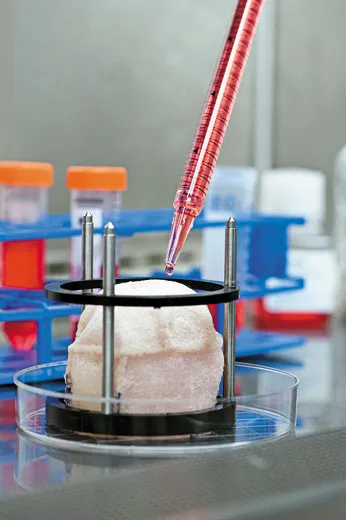
Anthony Atala works in the body shop of the future. He is the director of the Wake Forest Institute for Regenerative Medicine in Winston-Salem, North Carolina, and he and his colleagues use human cells to grow muscles, blood vessels, skin and even a complete urinary bladder. Much of the work is experimental and hasn’t yet been tested in human patients, but Atala has implanted laboratory-grown bladders into more than two dozen children and young adults born with defective bladders that don’t empty properly, a condition that can cause kidney damage. The bladders were the first lab-generated human organs implanted in people. If they continue to perform well in clinical tests, the treatment may become standard not only for birth defects of the bladder but also for bladder cancer and other conditions.
Atala and co-workers make replacement parts out of patients’ own raw materials. To produce a bladder, they remove a small piece of a patient’s organ and separate out muscle cells and urothelial cells, which line the urinary tract. They put the cells into lab dishes and bathe each type in a fluid that prompts them to multiply. After six weeks, there are enough living cells for an entire bladder. The researchers then pour the muscle cells onto the outside of a scaffold made of collagen, the protein in connective tissue, and polyglycolic acid, a material used in absorbable sutures. Two days later, they coat the inside of the scaffold with urothelial cells. The new bladder is nurtured in an incubator that mimics body conditions, allowing the cells to grow and knit together. The bladder is then implanted into a patient, where the scaffold gradually dissolves. The researchers have standardized the bladder-growing procedure, Atala says with a smile, and now make “small, medium, large and extra-large sizes.”
Regenerative medicine’s once-wild ideas are fast becoming reality. Late last year, Organovo, a biotech company in San Diego, began distributing the first commercially available body-part printer. Yes, you read correctly: a printer for body parts. Using the same idea as an ink-jet printer, it jets laser-guided droplets of cells and scaffold material onto a movable platform. With each pass of the printer head, the platform sinks, and the deposited material gradually builds up a 3-D piece of tissue. Regenerative medicine laboratories around the world have relied on the printer to generate pieces of skin, muscle and blood vessels. Atala’s lab has used the technology to construct a two-chambered mouse-size heart in about 40 minutes.
Atala and his colleagues have also managed to fashion lab-built kidneys that produce urine when implanted into experimental animals. And within a few years, he says, human skin could be coaxed into growing in a lab and be given to burn victims and other patients who today must undergo painful skin grafts.
Organs grown outside the body will transform medicine, Atala predicts, but spurring repair and regrowth within the body will be just as important. He and other scientists foresee injecting healthy cells and growth-inducing molecules into diseased or injured lungs, livers and hearts, prompting them to regenerate. Then there’s the ultimate challenge: Could a patient someday regrow an entire limb?
“It is not outside the realm of possibility,” Atala says. “If a salamander can do it, why can’t a human?” Scientists are getting closer to understanding the subtle genetic and physiological processes that allow salamanders to regenerate their limbs from scratch. More clues are coming from lab mice that have a genetic mutation that allows them to partially regrow severed digits.
Will doctors 40 years from now be able to help humans regrow severed spinal cords, damaged hearts or even lost limbs? Atala says he is optimistic: “The things that are possible today were a dream 20 years ago.”
Gretchen Vogel lives in Berlin and writes for Science.