Kill All the Mosquitoes?!
New gene-editing technology gives scientists the ability to wipe out the carriers of malaria and the Zika virus. But should they use it?
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/82/81/8281f96c-50c8-4be0-b36a-29d343a27555/jun2016_i03_mosquitos.jpg)
To the naked eye, the egg of the Anopheles gambiae mosquito is just a dark speck, but under a 100-power microscope, it shows up as a fat, slightly curved cucumber, somewhat narrower at one end. In the wild, it is typically found in shallow, sunlit puddles in sub-Saharan Africa, but it can survive in any number of wet places at around 80 degrees Fahrenheit. In a laboratory in London, behind three sets of locked doors enclosing negative-pressure containment vestibules, Andrew Hammond, a doctoral student in molecular genetics, picks up a clump of Anopheles eggs on a small paintbrush and lines them up on a microscope slide. Hammond looks for the narrow end, where the germ line cells that will form the next generation are located. With delicate nudges of a joystick, he maneuvers a tiny needle through his field of vision until it just penetrates the egg membrane, and the click of a button releases a minute squirt of DNA. Whether the genetic material reaches and binds to its target region is then a matter of luck, and luck is, generally, with the mosquito. Hammond’s success rate, of which he is very proud, is around 20 percent.
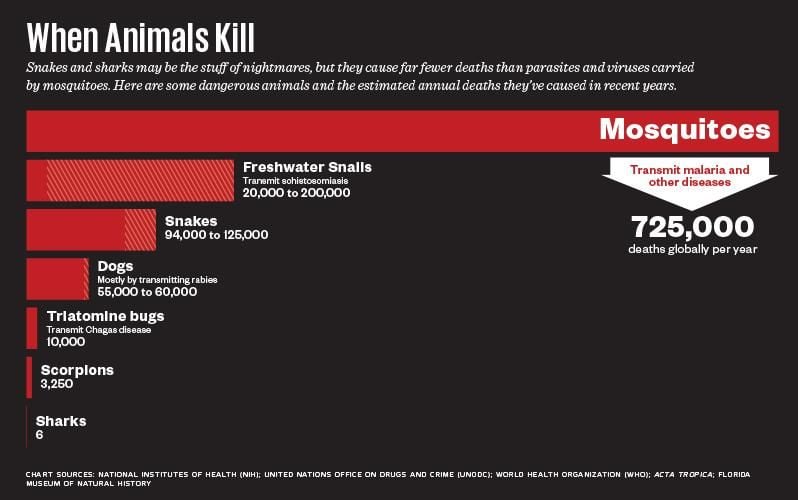
A. gambiae has been called the world’s most dangerous animal, although strictly speaking that applies only to the female of the species, which does the bloodsucking and harms only indirectly. Its bite is a minor nuisance, unless it happens to convey the malaria parasite, Plasmodium falciparum, for which it is a primary human vector. Although a huge international effort has cut malaria mortality by about half since 2000, the World Health Organization still estimates there were more than 400,000 fatal cases in 2015, primarily in Africa. Children are particularly susceptible. The Bill and Melinda Gates Foundation prioritized malaria in its more than $500 million commitment to fight infectious disease in developing countries. A portion of that money ends up here, in the laboratory of Andrea Crisanti at Imperial College, London, a short walk from Harrods.
Crisanti, a tousled, sad-eyed man with a gentle smile, was trained as a physician in Rome. Later, studying molecular biology in Heidelberg, he developed his lifelong interest in malaria. He set out on the trail of A. gambiae some 30 years ago, after he concluded that the best way to eradicate the disease was to attack the mosquito rather than the parasite. “The vector is the Achilles’ heel of the disease,” he says in his soft Italian accent. “If you go after the pathogen [with drugs], all you are doing is generating resistance.”
Humans have been at war with members of the family Culicidae for over a century, since the pioneering epidemiologist Sir Ronald Ross proved the role of Anopheles in malaria and U.S. Army Maj. Walter Reed made a similar discovery about Aedes aegypti and yellow fever. The war has been waged with shovels and insecticides, with mosquito repellent, mosquito traps and mosquito-larvae-eating fish, with bed nets and window screens and rolled-up newspapers. But all of these approaches are self-limiting. Puddles fill up again with rain; insects evolve resistance to pesticides; predators can eat only so much.
By the time Crisanti joined Imperial College, in 1994, molecular genetics had suggested a new approach, which he was quick to adopt, and in which his lab is now among the most advanced in the world. Scientists had discovered how to insert beneficial mutations—such as the gene for Bt, a natural insecticide—into agricultural crops such as corn. Why not, then, create a lethal mutation and insert it into the DNA of a mosquito? One problem was that mosquitoes weren’t bred in a factory, as commodity corn increasingly is. In the wild, mosquitoes mate randomly and propagate by Mendelian inheritance, which dictates that a mutation spreads slowly, if at all. Unless the man-made mutation conveyed some strong evolutionary advantage—and the whole point was to do the opposite—it would most likely disappear.
In 2003, Austin Burt, a colleague of Crisanti’s at Imperial College, suggested a solution: coupling the desired mutation with a “gene drive” that would overwrite the ordinary processes of inheritance and evolution. Recall that genes are spelled out by DNA sequences woven into chromosomes, which come in pairs (23 pairs in a human, 3 in a mosquito). A “gene drive” involves copying a mutated gene from one chromosome onto the other member of the pair. The key is that when the pairs split to form the eggs and sperm, it won’t matter which chromosome gets passed along—the engineered gene will be there either way. Thus a single mutation would, in theory, be “driven” into practically every mosquito in a breeding population.For the next dozen years, Crisanti, working with a senior research fellow named Tony Nolan and others, obsessively pursued variations of this approach, designing one gene mutation that would render females sterile and another that would lead to a huge preponderance of males. The challenge was creating the particular gene drives that duplicated those mutations—a tedious, years-long process of constructing custom DNA-snipping enzymes.
Then, in 2012, the UC Berkeley researcher Jennifer Doudna and her colleagues developed a revolutionary new technique for editing DNA. Researchers had known for years that certain genes in bacteria had short, repeating chunks of DNA. (CRISPR stands for “clustered regularly interspaced short palindromic repeats.”) When a virus invaded, the bacteria copied part of the virus’ genetic code, slotting it into the spaces between the repeating CRISPR chunks. The next time the bacteria saw that piece of code, an enzyme called Cas9 would guide its RNA to exactly that sequence in the gene of the invading virus. It would cut out the DNA with incredible precision and fuse the strand back together. Doudna and her colleagues harnessed this process in the lab, using it to quickly and easily edit any part of a gene they targeted. The following year, separate teams led by MIT bioengineer Feng Zhang and Harvard’s George Church showed it would work in living cells.
It was the universality as well as the accuracy that set CRISPR-Cas9 apart from other gene-editing techniques. Unlike the custom enzymes Crisanti and his team had been painstakingly building, Cas9 seemed to work in any type of cell. Researchers saw implications for treating genetic disorders, for improving agriculture—and for more sinister applications, such as creating biowarfare agents. CRISPR also brought Crisanti’s dream a giant step closer to reality. Now, he and his team could program Cas9’s guide RNA to pinpoint any part of a gene and transfer over the material they wanted to copy.

If Crisanti’s approach works, you could, in theory, wipe out an entire species of mosquito. You could wipe out every species of mosquito, although you’d need to do them one at a time, and there are around 3,500 of them, of which only about 100 spread human disease. You might want to stop at fewer than a dozen species in three genera—Anopheles (translation: “useless,” the malaria mosquito), Aedes (translation: “unpleasant,” the principal vector for yellow fever, dengue and Zika) and Culex (translation: “gnat,” responsible for spreading West Nile, St. Louis encephalitis and other viruses).
For thousands of years, the relentlessly expanding population of Homo sapiens has driven other species to extinction by eating them, shooting them, destroying their habitat or accidentally introducing more successful competitors to their environment. But never have scientists done so deliberately, under the auspices of public health. The possibility raises three difficult questions: Would it work? Is it ethical? Could it have unforeseen consequences?
**********
The feasibility question is being studied in Crisanti’s London lab, where the injected eggs will hatch into larvae. The ones harboring the mutation are identified by a “marker” gene, which glows under a microscope when viewed in certain lights. The mutants of interest are then returned to the warm, humid air of the mosquito rooms, to stacked trays with walls of white plastic mesh. On one side, there’s a long socklike tube, ordinarily tied in a knot, through which researchers can insert an aspirator to gently vacuum up specimens. If you hold your hand nearby, the females, sensing the nearness of blood, gather on that side. When it’s time for their blood meal, which will nourish the hundred or so eggs a female will lay at one time, an anesthetized mouse is laid belly-down on the cage roof, and the females fly up to bite it through the mesh. (The males, which live on nectar and fruit in the wild, feed on a glucose-water solution, wicked up from a small glass bottle.) These insects live up to a month longer in the controlled environment of the cages than in the wild, where they often don’t survive more than a week or two.
The next phase of the research takes place in Perugia, Italy, home to one of the world’s oldest universities, founded in 1308, and to a small, elite research consortium, Polo d’Innovazione Genomica. A few miles from the winding alleys of the medieval hilltop village, in a glass-walled building on a stark windswept plaza, is Polo’s secure lab, with six ceiling-high “field cages,” each with an area of 50 or 60 square feet. Signs on the doors warn away visitors who might have been exposed to malaria, since they could infect an escaped mosquito if it bit them. The air inside is tropical. Instead of live mice, females are fed on small dishes of bovine blood, warmed to body temperature and covered with paraffin, to give them something to land on. The females are attracted to the pheromones in human sweat, especially from the feet. Lab workers say they sometimes wear their socks all weekend and bring them to work on Monday to rub on the feeding dishes.
Inside, the lighting changes to simulate a 24-hour tropical day, and environmental cues trigger the swarming behavior that is crucial to mating. “That is how many insects mate,” explains the chief entomologist, Clelia Oliva. “The males swarm, and the females fly through the swarm and find a mate, and they come together in the air. If you cannot replicate that, you cannot determine if your line is going to succeed in the wild.” An escapee from one of the cages flits past Oliva as she is talking, and she dispatches it with the slap she perfected while studying mosquitoes on Reunion Island, in the Indian Ocean.
Researchers are skeptical about whether it is even possible to wipe out mosquitoes. “Global elimination of an entire species, I think, is a little far-fetched,” says Steven Juliano, an ecologist at Illinois State University. But, he adds, “I think they have a good chance of reducing local populations, maybe even eradicating a species in a locality.”
Something like that has been done with other creatures. Starting in the 1950s, the American entomologists Edward F. Knipling and Raymond C. Bushland eliminated the screwworm, an agricultural pest, from the United States and much of Central America. Their approach, called “sterile insect technique,” involved breeding and hatching millions of flies, sterilizing the males with low-level gamma rays, then releasing them in numbers sufficient to swamp the wild population. Females that mated with the sterile males produced infertile offspring. It took decades, but it worked—the two men were awarded the World Food Prize in 1992—and the same technique now is used to contain outbreaks of the Mediterranean fruit fly.
But when the sterile insect technique was tried against mosquitoes, the results were mixed. It requires that the released males compete successfully with their wild counterparts in mating, and there is evidence that in mosquitoes, the same radiation that makes them sterile may also impair their mating behavior. Whatever female mosquitoes are looking for in a mate, these males seem to have less of it.
So researchers have also been looking at variants of sterile insect technology that don’t require radiation. A pilot project has begun in the city of Piracicaba, in southeastern Brazil, by the British biotech company Oxitec. The target insect is A. aegypti, the main culprit in spreading yellow fever, dengue and other viral diseases, and the work has taken on greater urgency in the last six months, because A. aegypti also is a vector for the Zika virus, blamed for an outbreak of terrifying birth defects in the Americas.
In Oxitec’s program, male larvae bred with a lethal mutation are raised in water dosed with the antibiotic tetracycline, which inactivates the lethal gene. When those males mate with wild mosquitoes, their offspring, deprived of tetracycline, die before they can reproduce. CEO Hadyn Parry claims “greater than 90 percent suppression of the wild population” in five studies that covered relatively small areas in Brazil, Panama and the Cayman Islands. Now the company wants to expand to the subtropical U.S., and it recently passed a key regulatory hurdle to bring the program to the Florida Keys.
Oxitec’s technology predates CRISPR, and it doesn’t use a gene drive. Its goal is not to exterminate Aedes, but to reduce the local population to where it can no longer serve as a vector for human disease. That is, of course, a temporary solution to a perennial problem. Mosquitoes don’t usually travel more than a few hundred yards from where they hatch, but people do, and they can take yellow fever with them. And the mosquitoes themselves can travel the globe on airplanes and ships. Aedes albopictus, the “Asian tiger mosquito,” arrived in the Western Hemisphere a few years ago, possibly in a shipment of tires, and spreads many of the same diseases as A. aegypti. So even if the Oxitec program succeeds, it will likely need to be repeated at intervals. “You begin to see why Oxitec is a business,” one American entomologist said dryly.
**********
The Buzz About Altered Bugs
How the revolutionary technique CRISPR-Cas9 gives scientists the ability to insert an infertility gene into a mosquito—so the gene “drives” into a population, eventually causing its demise:
Engineering the Gene
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/02/0a/020af78c-c91a-46a5-9f1e-90071b9bfc5b/gene-drive-final1-wr-v1.jpg)
Scientists create genetic code that disrupts reproduction in female mosquitoes and inject the custom DNA into a fertilized mosquito egg.
Mutant Mosquito
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/2a/15/2a15105c-24f2-4c13-a401-3d8546e17819/gene-drive-final2-wr-v1.jpg)
As the insect develops, the engineered gene is incorporated into the cells that generate sperm in males and eggs in females.
Mosquito vs. Human Chromosomes
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/2c/2e/2c2e9506-7909-477c-b81c-be39d0dba753/gene-drive-final3-wr-v1.jpg)
Mosquitoes have three pairs of chromosomes total (humans have 23), but a sperm or egg cell contains just one member of each chromosome pair. In an altered insect, the engineered gene (in orange) is now part of a chromosome in the sperm or egg.
Pairing of Chromosomes
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/61/e4/61e4d5fc-fd74-45fb-af66-796669d518eb/gene-drive-final4-wr-v1.jpg)
When an altered mosquito mates with a wild insect, their offspring’s chromosomes are paired. The engineered DNA comes with a highly targeted editing enzyme, which helps insert the alteration into the wild chromosome. From left to right:
-
A mosquito inherits one chromosome from each parent.
-
The Cas9 enzyme snips out a gene on the wild chromosome.
-
The wild chromosome repairs itself, with the
altered gene as a template. -
Now both chromosomes in the pair carry the mutation.
Down the Generations
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/99/bf/99bf06ce-9e7d-4d9d-a141-c665d420c11a/gene-drive-final5-wr-v1.jpg)
With the altered gene on both chromosomes, it will become more prevalent in a population (in contrast to natural mutations that lack the gene drive mechanism). The altered gene (shown as a circle, right) is carried by male mosquitoes (orange), which remain fertile. Females that inherit the alteration from both parents are sterile.
**********
There’s not much doubt that eradicating Anopheles gambiae and Aedes aegypti would save many lives, and for most people that’s a good enough reason to do it. “I don’t think the world would be a worse-off place if local populations of these species were eliminated,” Juliano says, “and it wouldn’t bother me any more than eliminating the smallpox virus.” Even the great conservationist E.O. Wilson, the world’s most famous entomologist, has said he wouldn’t mourn A. gambiae. “Keep their DNA for future research,” he says, “and let them go.”
Still, there are voices calling to proceed slowly. “If we were to intentionally set out to cause the extinction of a species, we should think about that,” says Henry Greely, a Stanford law professor and bioethicist. “I would want there to be some consideration and reflection, and a social consensus, before we take that step.” His argument is based partly on the slippery slope: If mosquitoes, then why not rats? “I’m not sure I care if mosquitoes suffer, if they can suffer. But mammals or birds, I do care.”
But suppose the target were the malaria parasite itself, which as a single-celled protozoan has even a smaller claim on our sympathy than an insect? At UC Irvine, Anthony James, a geneticist, has been working since the 1980s on breeding mosquitoes that, while viable themselves, do not transmit P. falciparum. The virus has a complicated life cycle that takes up to three weeks to move from the mosquito’s gut to its circulatory system to the salivary glands, from which it is transmitted. James realized that if he could endow the mosquito with genes that produce antibodies to P. falciparum, he could destroy the parasite without having to kill even one insect. He created the gene for the antibodies, but he needed a way to make it spread in the wild.
Then he heard about CRISPR-Cas9—in particular the work being done at UC San Diego by a molecular biologist named Ethan Bier, who recently put a mutation into fruit flies. Bier allows that some situations might warrant removing a genus like A. aegypti from a vast area of the world where it isn’t native. Whenever possible, though, he prefers less-invasive methods. “I like this approach, of modifying the mosquitoes rather than rendering them extinct,” says Bier. “We’re doing enough of that already. As a human being I don’t want to be involved in the eradication of a species, even an insect.” James has successfully engineered the antibody-producing genes and is working on the gene drive. He could have insects ready for field tests in a matter of months but can’t predict how long the approval process will take. “We’re not about to do anything foolish,” he says.
**********
If society chooses to eliminate one or more species of mosquito, what are the downsides? Mosquitoes play a critical role in a few environments, such as the Arctic tundra, where they hatch out by the billions over a short period and are a significant food resource for birds. In most other places, biologists believe, the ecosystem could survive the loss.
Still, according to Nolan, “Our goal is not to eliminate malaria mosquitoes from the face of the earth. If we succeed, people won’t even notice. There will be plenty of mosquitoes out there.”
It’s possible, even likely, that another species would take the place of the mosquitoes we exterminated. For instance, A. aegypti could be replaced by a mosquito from the Culex pipiens species complex. Culex, which is a vector for the West Nile virus, “does very badly when Aedes is present,” Juliano notes, but it might be expected to thrive in its absence. On the other hand, the newcomer might be a relatively harmless species; the ecological niche for mosquitoes doesn’t require them to carry diseases fatal to human beings. In the long term, the pathogens could evolve to be spread by the mosquitoes that are still around, but there’s plenty of time for humans to worry about that.
The larger concern, arguably, is over the use of CRISPR itself, and the awesome power it unleashes over the environment. “We can remake the biosphere to be what we want, from woolly mammoths to nonbiting mosquitoes,” Greely muses. “How should we feel about that? Do we want to live in nature, or in Disneyland?” Another fear is that CRISPR puts a potential weapon in the hands of terrorists, who could use it to engineer epidemics. “Just as gene drives can make mosquitoes unfit for spreading the malaria parasite, they could conceivably be designed with gene drives carrying cargo for delivering lethal bacterial toxins to humans,” warns David Gurwitz of Tel Aviv University.
The National Academies of Science, Engineering and Medicine thought enough of the threat to convene a conference last fall on the implications of gene drive technology for biosecurity. But many scientists think this is an overblown concern (along with the other horror-movie scenario, of a high-school student in his basement using CRISPR to make a dog that glows in the dark). “A gene drive in a mosquito would make a very poor bioweapon,” says Kevin Esvelt, an ecologist at MIT, who has written extensively on the subject. “They are slow [compared with disseminating a deadly microbe], they are easy to detect, and it’s straightforward to build a reversal mechanism.”
But Esvelt has other ethical concerns about using CRISPR technology on animals: “We will have engineered the ecosystems of people elsewhere in the world without their knowledge or consent. We go from the default assumption that the things we engineer will not spread, to assuming they will. Normally you can make any kind of fruit flies you want—natural selection will wipe the floor with them. But as soon as you’re thinking of a gene drive technology, you have to assume whatever you’re making will spread once it gets outside the lab. Human error will win out, if not deliberate human action.”
Yet Esvelt himself is already thinking about whether and how to someday use a CRISPR gene drive in a mouse, the main animal reservoir of Lyme disease—and a mammal. He would engineer a local population to carry antibodies for the bacteria that cause Lyme. (The disease spreads from mice to humans through tick bites.)
If CRISPR works in a mouse, it will almost certainly work in a human being. The least controversial application would be for inherited diseases such as muscular dystrophy—which would most likely involve repairing the somatic (non-reproductive) cells of a child or an adult. But Chinese scientists just announced the results of their second study of CRISPR in human embryos. (They used nonviable embryos from fertility clinics.) The results revealed “serious obstacles” to the approach, but the technology is fast improving. Harvard scientists, for instance, recently modified the CRISPR method so it can change a single letter of the genetic code, making it easier to prevent diseases like Alzheimer’s and breast cancer. CRISPR also opens the Pandora’s box of editing the germ line cells that pass on their genetic material to succeeding generations. This could be of enormous benefit to a small number of people who carry genes for disorders such as Huntington’s disease. More problematically, it could encourage parents to custom-build their offspring, deleting genes that are unwanted but not life-threatening (for lactose intolerance, say), or adding ones that convey traits such as athletic ability, longevity—or intelligence.
This possibility has given rise to a lot of op-ed angst about “playing God,” which certainly should be taken seriously. Leaving aside the philosophic objections, the practical downside is that we don’t know all of the genes that will actually make someone smarter (or taller, stronger, healthier, faster and so forth) and the only way to find out for sure is to try different combinations on various embryos and wait for them to grow up. By that time, if we got it wrong, it would be too late to fix, not least for the humans who were the unwitting subjects of the experiments.
That, in the eyes of most ethicists, is an insurmountable problem. An International Summit on Human Gene Editing in Washington, D.C. last December aired many of these issues, revealing a split between the medical community, which wants to help patients in the here and now, and some researchers, who worry about the implications of the tabloid headline announcing the birth of the first Frankenbaby.
Meanwhile, mosquitoes flit about the villages and cities of central Africa, land silently on sleeping children and bite. The fight against malaria has made much progress in the last decade, but at a huge cost that may not be sustainable indefinitely. In the Western Hemisphere, the threat of Zika has led to extraordinary measures, including warnings in whole regions of South and Central America for women to consider postponing childbearing. This summer will tell us if the disease will strike in the parts of the U.S. where two Aedes species live—Florida and a strip of the Gulf Coast that is likely to expand as the winters warm in a changing climate. (The second of those two American Aedes species, A. albopictus, is a confirmed carrier of the virus and can be found as far north as New England.) Public-health officials are already bracing for the possibility of a spate of babies with the devastating diagnosis of microcephaly and associated brain damage. It was human transportation technology that spread these diseases across the globe. Now technology is offering a way to contain them, or even defeat them altogether, at the risk of unleashing powerful forces whose effects we can only dimly predict.
Will we do it—we humans, the species with the relentless appetite for knowledge? The fruit of that particular tree has never been left uneaten for very long. Crisanti, for his part, is ready to pick it. “I want to see malaria wiped out in my lifetime,” he says softly. He is 61.
Related Reads

Mosquito: The Story of Man's Deadliest Foe




