What Is Killing the Bats?
Can scientists stop white-nose syndrome, a new disease that is killing bats in catastrophic numbers?
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/bats-researcher-checking-wings-631.jpg)
Inside the gaping mouth of Mammoth Cave, hibernating bats sleep in permanent twilight, each huddled in its own limestone crevice. Every fall, these big brown bats (Eptesicus fuscus) squeeze their furry bodies into nooks in the cave walls, where they enjoy protection from the bitter wind and the waterfall that sprays across the entrance. But there’s little a snoozing bat can do about a persistent scientist.
“Just...let...go...with...your...feet,” coaxes Brooke Slack, a biologist at the Kentucky Department of Fish and Wildlife Resources, as she stands on tiptoes and reaches with gloved hands to pry a bat from the wall.
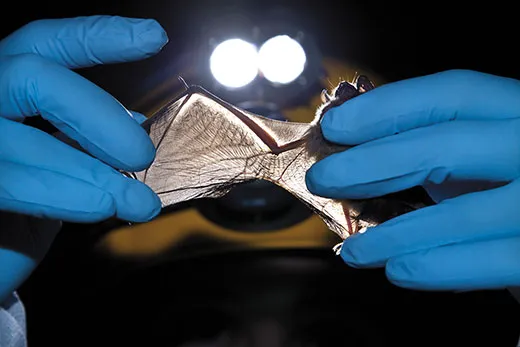
The bat, visible by the light of her headlamp, lets out a stream of tiny, infuriated shrieks, baring its sharp white teeth in protest. Slack gently loosens the bat’s claws from the rock and slips the four-inch-long animal into a brown paper bag. On this gray December afternoon, Slack and her colleague, a Northern Kentucky University microbiologist named Hazel Barton, are pressing this unlucky bat into service for its species.
Mammoth Cave, the longest known cave in the world, stretches at least 390 miles under the forests of southern Kentucky, and its twisting tunnels have fascinated explorers, scientists and tourists for well over a century. Slack and Barton have come for a different reason: the cave is a front line in the most precipitous decline of North American wildlife in living memory.
With a half-dozen grumpy bats bagged, Slack, Barton and several co-workers lug their gear to the cave’s Rotunda Room, where the limestone forms a grand domed ceiling. On summer days, this natural underground chamber is packed with tourists, but today the scientists have the place to themselves. Clad in disposable white Tyvek suits to avoid tracking microbes into or out of the cave, Slack holds each protesting bat while Barton clips samples of hair and swabs faces and wings.
“Look at you, with your dirty, dusty little face,” Barton coos, shining her helmet lamp on one screaming bat.
Barton and Slack are good friends, and they work together often even though they have different passions. Barton is interested in bats because they live in caves. Slack is interested in caves because they’re home to bats. Barton has a map of South Dakota’s Wind Cave tattooed on her arm. Slack has a tiny silhouette of a bat tattooed behind her ear.
They both know that somewhere in this cave, even on these bats, may lie spores of the fungus Geomyces destructans, which is devastating hibernating bat populations in the Northeastern United States. The fungus appears to be the cause of a disease called white-nose syndrome, which has killed more than a million bats in the past four years. It even threatens some of the continent’s most abundant bat species with extinction.
Mammoth Cave has nearly 500,000 visitors per year, any one of whom could transport spores in or out. So far, despite painstaking searches by Slack and her crew, the fungus has not been found. But the disease has been confirmed in neighboring Virginia, West Virginia and, most worrisome, in a Tennessee cave only 80 miles from Mammoth.
“Oh, look at this,” Slack says to her colleagues. They hear the note of concern in her voice, and the silence is immediate and thick. As headlamps turn toward her, Slack stretches out a bat wing, its thin membrane marked by two half-inch tears. They could be from a run-in with an owl, or a barbed-wire fence. Or they could be a sign that white-nose syndrome has crossed the state line and arrived in Mammoth.
The other bats collected today will be returned, ruffled but unharmed, to their hibernation perches, but this one will be euthanized for laboratory tests. Reluctantly, Slack and Mike Armstrong from the U.S. Fish and Wildlife Service do the deed with a vial of the chemical isoflourine. “Sorry, little girl,” Armstrong says. One bat sacrificed, in hopes of saving another million of its kind.
Barton has just spent eight days squeezing her lanky frame through unexplored sections of Lechuguilla Cave, a southern New Mexico cave thought to be the deepest in North America. Access is restricted to protect Lechuguilla’s delicate crystals and stalactites as well as its relatively undisturbed microbial community. Though Barton is an expert caver, more than a week in tight passages has tested even her stamina, leaving her knees sore and her gait stiff. But she saw a part of the world that’s never been seen before.
She grew up in Bristol, England, in a family she describes as “not the slightest bit outdoorsy.” When she was 14, she participated in a required high-school course that included rock-climbing, kayaking, horseback riding and a day of caving. “Everything terrified me but the caving,” she says. “In the cave, I stayed in the back of the group thinking, ‘I love this. This is cool.’”
Barton began to explore the caves near her hometown, caving with friends several times a week (“My mother would say, ‘You can’t go caving now! It’s dark!’” she says with a laugh). As her curiosity and enthusiasm grew, she began exploring more difficult and distant caves.
She had also been fascinated by microscopic organisms ever since hearing BBC-TV naturalist David Attenborough marvel about the complexity of life in a single drop of water. When she was 14, Barton swept her hair against a petri dish of nutrients in science class. “By the next day, all kinds of disgusting things had grown out of it,” she remembers with a grin. After studying biology at the University of the West of England, she moved to the University of Colorado to pursue a PhD in microbiology.
A collaborator, Norman Pace, suggested she study the microscopic life in caves, which scientists knew little about. “There aren’t many microbiologists who can go where you go,” Pace told her. Barton didn’t want caving—her hobby—to become her job, but eventually she relented and began to plumb caves in Mexico, Guatemala, Belize, Venezuela and throughout the United States for signs of microbial activity. Caves, she has found, are swarming with microbes adapted to life without photosynthesis. She has identified microbes that can digest industrial chemicals and others with antibiotic properties—organisms that she and other researchers are studying for their potential to treat drug-resistant human diseases.
Barton’s experience schooled her in the tenacity of these tiny life-forms. For her PhD research, she studied a bacterium that infects the lungs of cystic fibrosis patients, and she came to think of caves as somewhat like human bodies—complex places that host a vast variety of organisms, each adapted to its environment in a different way. Yet when Barton heard that a bat-killing fungus had managed to spread from caves in New York State all the way to West Virginia in just two years, even she was surprised by its velocity.
“if you sat down and thought, ‘What would I design to kill bats, and how would I design it?’ and you took time to think about the worst possible combination of factors that a pathogen would have, this would be it,” says Barton.
Because G. destructans thrives in cool temperatures, it attacks bats while they hibernate for the winter, when their immune systems are effectively shut down. The fungus may spread from bat to bat, and when the animal colonies disperse in the spring, the fungus may persist in cave sediment, poised to infect the next winter’s arrivals. Bats with white-nose syndrome rouse more frequently from their winter torpor, which causes them to waste precious body fat at the coldest time of the year. (In what’s been dubbed the “itch and scratch” hypothesis, some scientists posit that the bats are disturbed by the fungus, which accumulates on their muzzle and wings.) The fungus also infects the bats’ delicate wing membranes, eating away at the skin until the wings resemble torn, crumpled tissue paper.
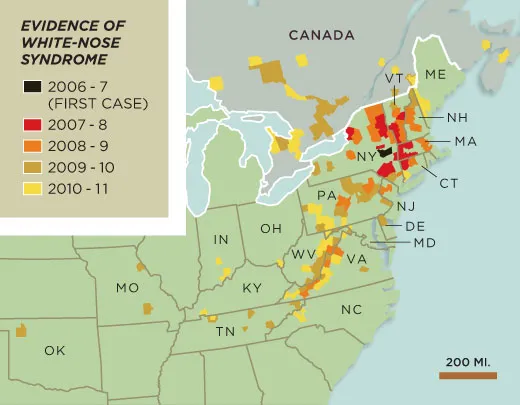
The disease was discovered in early 2007, when bats in upstate New York started behaving oddly. Instead of hibernating through the winter, they flew into neighborhoods during the day, wandering dangerously far from their caves. “There would be three feet of snow and it would be 20 degrees—not bat-flying weather—and you’d see bats flying out and taking off into the distance,” says Al Hicks, then a wildlife biologist for the New York State Department of Environmental Conservation. “You’d know every darn one of them was going to die. It was awful.”
Later that winter, during a routine cave survey, New York State biologists found thousands of dead bats in a limestone cave near Albany, many encrusted with a strange white fuzz. During the winters that followed, dead bats piled up in caves throughout the Northeast. The scientists would emerge filthy and saddened, with bat bones—each as thin and flexible as a pine needle—wedged into their boot treads.
By the end of 2008, wildlife-disease researchers had identified the fuzz as a fungus new to North America. Today the fungus has spread to 19 states and 4 Canadian provinces, and infected nine bat species, including the endangered Indiana and gray bats. A 2010 study in the journal Science predicted that the little brown bat—once one of the most common bat species in North America—may go extinct in the eastern United States within 16 years.
“When it first hit, I thought, ‘OK, is there anything we can do to keep it within this cave?’” remembers Hicks. “The next year it was, ‘Is there anything we can do to secure our largest colonies?’ And then the next year it was, ‘Can we keep any of these colonies going?’ Now we’re asking if we can keep these species going.”
G. destructans also infects bats in Europe—but it doesn’t kill them, at least not in large numbers. G. destructans may have swept through European caves in the distant past, leaving only bats that could withstand the fungus. Researchers don’t know when and how the fungus made its way to North America, but they speculate that it may be so-called “pathogen pollution,” the inadvertent human transport of diseases—in this case possibly by a cave-visiting tourist—into new and hospitable habitats.
With their undeserved association with creepy folk tales, bats don’t have much of a constituency. But bat biologists say the consequences of the North American die-off stretch far beyond the animals themselves. For instance, one million bats—the number already felled by white-nose syndrome—consume some 700 tons of insects, many of them pests, every year. Fewer bats mean more mosquitoes, aphids and crop failures. A study published in Science this spring estimated that bats provide more than $3.7 billion in pest-control services to U.S. agriculture every year.
With G. destructans reaching farther each winter, Barton, Slack and an array of other biologists are racing to understand the fungus in time to contain it. Since scientists aren’t sure how easily people may spread the fungus, many caves have been closed, and tourists, recreational cavers as well as scientists are advised to clean their gear between trips underground. Barton and her students have shown that common cleaning products, such as Woolite and Formula 409, kill G. destructans without harming caving gear.
But even as Barton, Slack and their colleagues patrol the perimeter of the disease, they acknowledge that the syndrome is likely to continue its spread across the continent.
“Who’s going to live, and who’s going to die?” asks DeeAnn Reeder. “That’s the big thing I think about all the time.” Reeder, a biology professor at Bucknell University in central Pennsylvania, spends her days surrounded by white-nose syndrome. G. destructans thrives in nearby caves and mines, on many of the bats in her campus laboratories, and even on a set of petri dishes secured in an isolated laboratory refrigerator. Up close, the epidemic is more complicated than it first appears, for some bat species—and some individual bats—are proving more resistant than others. Reeder wants to know why.
Reeder never expected to study white-nose syndrome, but like Barton, she was perfectly prepared for the job. Fascinated by mammals since her childhood summers in the Sierra Nevada, she studied primate physiology and behavior before switching to bats. At first, the reasons were practical—bats were easy to catch and sample in large numbers—but “I just fell in love with them,” Reeder says. “They’re so tough. I’ve always said that nothing will take them down, that they’re completely resilient. And then we got this fungus,” she says, shaking her head. “It caught us all off guard—and it caught them off guard, too.”
After Reeder came to Pennsylvania in 2005, she outfitted her laboratory with a set of climate-controlled chambers designed to mimic natural cave conditions. She and her students had just begun to collect data on bat hibernation patterns when white-nose syndrome emerged. Suddenly, biologists all over the continent had questions about how bats behaved during hibernation, and Reeder was one of the only researchers well-positioned to answer them. “They’d say, ‘What do we know about hibernation?’ and I’d say, ‘Well, we know this much,’” says Reeder, holding a finger and thumb close together.
Like Barton and the rest of the small corps of researchers pursuing the disease, Reeder abruptly reoriented her career to deal with it. She and her students picked up the normally stately pace of science, running experiments in the field and lab as quickly as they could devise them. These days, the hallway outside her laboratory is crowded with worn backpacks and other scuffed field gear. “Sometimes I feel like a rat on an electrified grid,” she says with a laugh.
In Kentucky, Barton was also working overtime, sampling skin secretions and hair from bats in caves throughout the state. In her laboratory, she and her students cataloged naturally occurring antifungal compounds produced by bacteria and other fungi, identifying some compounds that might protect vulnerable bats from white-nose syndrome. But to test the most promising compounds, she needed something Kentucky didn’t yet have: sick bats.
When Reeder and Barton met at a bat conference in 2009, their complementary skills were obvious. “We spoke different languages, but it was clear that we needed to talk to each other,” says Reeder. Last fall, in southeastern Pennsylvania, Barton and several of Reeder’s students donned Tyvek suits and belly-crawled into the depths of one of the oldest limestone mines in North America. There, they trapped more than 100 infected bats and confined them in mesh enclosures with aerosolized antifungal compounds. They then left the bats alone to hibernate, hoping that some would survive until spring. They repeated the experiment in Reeder’s lab, applying the compounds to infected bats in her hibernation chambers.
On a mid-March afternoon, Reeder visits the four laboratory hibernation chambers that house the treated bats. The chambers, which resemble bulky refrigerators, held 128 bats last fall. Now, three of the four chambers are empty and quiet, shut down after the last of their bats died last month. In the corner of the dimly lit room, in the only operating chamber, a single bat survives—but it won’t live much longer. Through a small window, it’s possible to see its silhouette, hanging motionless from the metal rack inside. Its furry body is no larger than a human thumb.
Reeder and her students travel through the rolling Pennsylvania countryside, headed for the limestone mine where bats were caged last fall. The roadsides are dotted with gray stone houses and churches, reminders of the time when the area’s limestone provided shelter for people as well as bats. The mouth of the mine, tucked into a steep hillside above a two-lane highway, is blocked with a forbidding metal gate, designed to keep out vandals. Still, the cave is littered with beer bottles, and a message is unevenly spray-painted on the clammy rock: “This is great.”
But not for the bats in this mine, whose numbers have dropped from an estimated 10,000 two years ago to roughly 180 today. Reeder and her students zip up their Tyvek suits and pick their way through the fallen rocks on the mine floor, the beams of their headlamps cutting through the cool, misty half-dark. Little brown bats are hanging onto the rocks, alone or in twos and threes, their fur glistening with moisture. Here and there, a dead bat lies on the ground, the bodies hardly more substantial than dried leaves. The crew counts 35 live bats hanging just inside the mouth of the mine, almost half bearing visible signs of white-nose syndrome. All are far closer to the mine entrance than is normal for this time of year. Later, a few will flutter out of the mine, pale brown and reeling in the daylight.
The crew slips through a narrow horizontal slot on the side of the mine, crawling headfirst down a boulder-filled slope. There, more bad news awaits: the mesh cages have been vandalized by raccoons, and the treated bats inside have all either escaped or been eaten. An entire season of data lost—to raccoons! Among the researchers, the frustration is palpable, their reactions unprintable.
By the time she returns to the mouth of the mine, Reeder is philosophical. “I don’t do mopey very well,” she says. From her laboratory experiments, she already knows that the treatments they used can’t save bats from white-nose syndrome; at best, they may prolong their lives a little while. Perhaps different compounds, or higher concentrations of the same compounds, might boost survival rates, but those are questions for the next study.
In their search for patterns in the white-nose epidemic, Reeder and her students have found that bats in cooler conditions may have better survival rates. So it’s possible that humans could alter the temperatures in some mines—by changing the shape of entrances to direct airflow, for instance. In Tennessee, conservationists are already planning to build an artificial cave that can be kept fungus-free, and in New Hampshire, biologists are studying bats that hibernate in abandoned World War II-era bunkers, hoping that climate conditions inside will help some bats survive. The National Zoo has attempted to keep endangered Virginia big-eared bats alive in captivity, so far with limited success.
Even if such heroic measures can reduce the toll, many bat species will take generations to recover from white-nose syndrome. Thomas Kunz, a bat researcher at Boston University, is already preparing for these diminished populations. Since bats depend on each other’s body heat to warm their summer roosts, Kunz has devised artificial roosts—narrow crevices built of scrap lumber—that can be warmed efficiently by just a few bats.
“On my worst days, I feel like we’re working our tails off just to document an extinction,” says Reeder. “But somehow in really teasing apart all of this, in really understanding how they die and why, we may find something really important, something we didn’t predict, something that might help.”
This past winter, Brooke Slack and her crew conducted their annual survey of nearly 100 Kentucky caves. The early results were good: the bat she had euthanized in Mammoth Cave tested negative for white-nose syndrome, and the rest of their cave surveys came up clean. It looked as if Kentucky bats had, against the odds, made it through another winter fungus-free. But then white-nose syndrome showed up in southern Ohio, and Slack decided to recheck a few sites near the border, just to be sure.
On April 1, in a limestone cave in southwestern Kentucky, a researcher working with Slack found a little brown bat with white fuzz on its muzzle. They sent it to a laboratory, and a week later Slack got the news she’d anticipated, but dreaded, for the past three years: white-nose syndrome had finally arrived in Kentucky.
Now, Slack’s job is not only to slow the spread of white-nose syndrome, but also to learn as much as she can about the disease as it moves through her state—and her beloved bats. “There’s a sense of helplessness,” she admits. “But I don’t feel like we can say, ‘Well, we’ve got it, so we give up.’ We’ve got an obligation to move forward.”
Michelle Nijhuis has written about Atlantic puffins, Henry David Thoreau and last year’s Gulf oil spill for Smithsonian.