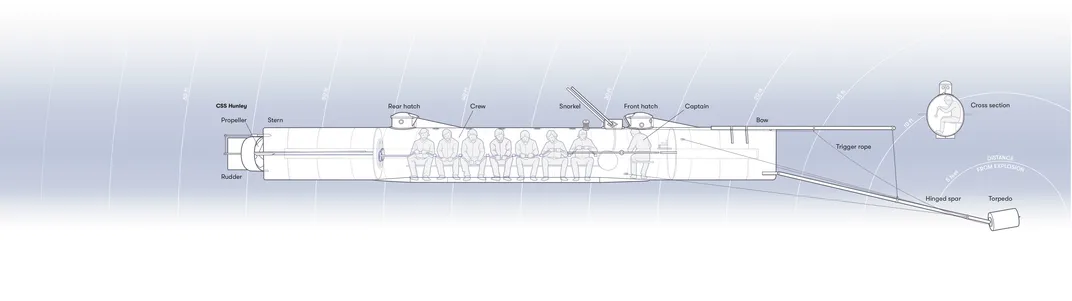
The dark hull of the submarine rose a few inches above the waterline. Pale moonlight glinted off the quiet ocean as small waves lapped against the hull. The submarine was about 40 feet long, cylindrical down most of its slim length, but with a tapered, wedge-shaped bow and stern that hinted at how quickly it could slice through the water. The deadlights, with their thick, imperfect, handmade glass, were the only sign that there might be a crew within.
The HL Hunley was lying in wait to the east of Charleston Harbor, off the coast of South Carolina. The submarine had been there for months, practicing for its crucial mission and waiting patiently for flat seas.
Its bow carried the source of its destructive power. A spar made of wood and metal was bolted to a pivot on the bottom corner of the boat’s leading edge, and at the far end of this spar was a copper cylinder the size of a keg: the boat’s torpedo. The torpedoes of the time were simple stationary bombs, very different from the modern, independent devices that can propel themselves through the water from a great distance. To complete its mission the Hunley would need to approach its target closely, then use this spar to press the charge directly against the side of the enemy’s hull.
On the deck of the USS Housatonic, sailors gazed out over a flat sea. The Housatonic was just one of many Union ships that had been prowling the waters outside Charleston for months, and tonight, like every other night, the silence was punctuated by the sounds of Union artillery.
The Hunley swam closer. It took hours to reach the ship.
A sailor on watch aboard the Housatonic spotted the sliver of dark metal hull exposed above the surface of the water and alerted others, but submarines were new technology and the men did not understand the deadly shape in the water. Their cannons weren’t positioned to hit an object so close by and down below, so they attacked with small-arms fire. But the submarine remained undeterred.
HL Hunley pressed its torpedo snugly against the Housatonic’s side. One of the three thin metal rods protruding from the leading face of the bomb depressed slightly against the wooden hull. The fragile wire holding the rod precariously in place snapped, freeing the coiled energy of the compressed spring that was firmly wrapped around the rod’s body. The rod smashed against the pressure-sensitive caps inside the charge, and they released a fiery inferno. As the black powder exploded, the copper casing ripped open, releasing the fearsome pressures of explosive black powder into the water and against the wooden hull of the Housatonic.

A spray of shattered wood planks burst upward from the deck of the ship. The submarine had hit its target, punching a lethal blow through the boat’s underbelly. The force of the blast rippled through the entire ship, and even the sailors at the bow nearly 200 feet away instantly understood that their vessel would soon be on the ocean floor.
As the crew scattered to save themselves, the metal hull of the submarine silently disappeared. Those in Charleston awaiting the return of the Hunley, hoping to celebrate its successful mission, never saw it again.
One hundred and thirty-six years later, in 2000, in a massive custom-built water tank, archaeologists clad in protective coveralls and wearing respirators sorted patiently through the muck and silt that had slowly filled the hull of the submarine as it lay on the bottom of the ocean floor. Accounts of the Hunley’s sinking had assumed horrific scenes of the men trying to claw their way through the thick iron hatches, or huddled in the fetal position beneath the crew bench in their agony. Sinkings of modern submarines have always resulted in the discovery of the dead clustered near the exits, the result of desperate efforts to escape the cold metal coffins; to sit silently and await one’s own demise simply defies human nature.
The crew of the Hunley, however, looked quite different. Each man was still seated peacefully at his station.
* * *
My research adviser at Duke University was Cameron “Dale” Bass, an associate professor of biomedical engineering, and Dale worshiped efficiency. To save time, every day he wore the same type of black polo shirt, with black or gray cargo hiking pants that zipped off at the knee, and the same heavy black lace-up combat boots. The students in Dale’s lab researched injury biomechanics: the various mechanisms by which human beings got injured and killed. About half the students worked on car crashes, and the other half, including me, focused on explosions.
In the Waves
In the Waves tells the story of how a determined scientist cracked the case of the first successful—and disastrous—submarine attack.

Before coming to Duke, I’d been a civilian engineer for the U.S. Navy, where I’d helped build underwater breathing systems. The Navy had sent me back to school to get a PhD in biomedical engineering, and in Dale’s lab, I was a natural fit to study underwater explosions. Most of my medical cases were from sailors injured during the Second World War. I combed through dozens of reports a day, looking for those in which a physician reported enough information to let me model the blast. The stories were usually the same: feeling of a sharp kick to the groin, with a stabbing pain in the gut. Sometimes they would immediately vomit blood, sometimes they would have sudden and uncontrollable bloody diarrhea. Both are signs of severe trauma to the intestinal tract. Sometimes they would start coughing up blood, a sign of damage to the lungs.
I would routinely get lost in the misery of the injuries, and it was during one of these depressing reveries that I heard the staccato thunk of Dale’s combat boots coming down the hall. All of us knew that sound. If the boots kept going, we kept working. But sometimes we heard the boots stop a few steps past a door, pause and then reverse. This meant Dale had an idea. Today, the boots stopped for me.
“What about the Hunley.” The words were delivered as a statement. “Can your fancy software model it?” he asked.
“Sure,” I responded, without any idea what he was asking. “I don’t see why not.” In grad school, unless you already have a damn good reason locked and loaded, the correct answer to such a question is always yes. Whatever he was talking about, assuming it was a boat of some kind, the Navy blast software I had been using could probably model it.
The boots proceeded down the hall.
I pulled up a new browser window on my computer and began to investigate what I had signed up for.
People are born with the instinct to fight against their own death, to struggle with their last breath against even the most unavoidable and uncompromising ends. And that universal instinct is why the Hunley case fascinates. The submarine is now housed at the Warren Lasch Conservation Center in North Charleston, South Carolina, where visitors are invited to “solve the mystery” at the end of their tour. The museum exhibits offer four theories: (1) that the torpedo damaged the hull and sank the boat, (2) that the crew was somehow trapped inside, (3) that the submarine collided with another object and sank, or (4) that a lucky shot fired from the crew of the Housatonic struck the captain.
Any of these theories would require that the crew members, with ample time to see their own deaths coming, chose to spend their last moments nobly in peace, seated at their stations. But that would defy human nature. Something killed these men. Something that left no trace on the boat or their bones.
If people near a bomb die, I always suspect some kind of effect from the bomb first. As I searched for information on the Hunley’s explosive charge, one image in particular kept appearing: a yellowed, faded scan of a cylinder, with hand-drawn lines and circles detailing its shape. “Singer’s torpedo,” proclaimed the large, old-fashioned calligraphy at the top of the image, with the more crucial information in a slightly smaller font below: “used for blowing up the Housatonic.”
According to this drawing, the Hunley’s charge contained 135 pounds of black powder. That is a lot of powder. It’s a lot of any kind of explosive.
The spar, attached to the bottom corner of the Hunley’s bow, had recently been conserved. What had initially seemed to be voluminous concretion, the accumulated crud of 13 decades underwater, had turned out to contain the peeled-back shards of the torpedo casing itself. I sat staring at photos of the beautiful, shiny copper ribbons. The bomb had to have been firmly attached to the end of the spar for those strips to be peeled back so cleanly. The spar was 16 feet long—which had to be the distance between the boat and the bomb.
At some point, the sun had set, and I realized I was destroying my potato-chip cache because I was starving. I closed my laptop, leaving open the browser windows so I could continue to stare at the pictures and articles later from home, over a burrito. I made my way out of the building, past the doors of open offices filled with other students still working into the night. As I swung my leg over my motorcycle, parked on the sidewalk outside the lab, I decided I could spare a few weeks to calculate the crew’s oxygen supply and determine whether suffocation was a realistic theory.
I have never so drastically underestimated the time it would take to solve a problem.
The next day I had the browser windows still open on my laptop, ready and waiting for Dale’s inevitable appearance in my office. “Well?” he asked. I angled the laptop screen toward him.
“This is the charge. One hundred thirty-five pounds of black powder.” I flipped to a second window. “This is the end of the spar. The charge was made of copper. It was still attached. The spar was 16 feet long.”
A third browser window. “These are the remains.” The image showed a neat, color-coded row of skeletons inside the hull of the submarine. Each color represented the remains of one individual, and each individual’s remains were crumpled in place at his battle station inside the boat.
“Nobody tried to escape. They died where they sat.” A grin spread slowly across Dale’s face.
* * *
Before I could begin to figure out whether the crew had somehow been killed or injured by their own massive bomb, I had to evaluate other theories that could explain their deaths. Had the men, for instance, suffocated inside the closed hull?
I was reasonably certain that suffocation, a term that specifically describes lack of oxygen or cessation of breathing, would not have caused the deaths of the Hunley crew. They could have asphyxiated, a more general term that would include the effects of carbon dioxide. But their bodies’ painful symptoms would have warned them that their demise was imminent, giving them time to try to escape.
Carbon dioxide is normally a tiny 0.04 percent of each breath that we inhale. As the percentage starts to climb, more and more CO2 is driven into the bloodstream. At around 5 percent, a person starts to notice what underwater divers in one experiment politely labeled “distracting discomfort.” The pain and discomfort escalate as the CO2 levels rise because the blood itself becomes increasingly acidic. Receptors in the brain sense the increase in acidity and try to counteract it. The blood vessels on the surface of the brain dilate in an attempt to transport the acidity away from the sensitive neurons; this dilation causes a headache. The brain increases the breathing rate and the heart rate and expands all the blood vessels, trying desperately to increase the amount of blood being pumped past the lungs so they can process and eliminate the deadly gas. In the end stages of carbon dioxide exposure, the acid in the veins begins to chemically break down the myriad enzymes and proteins that control bodily functions on a cellular level.
To do the math on the breathing gases, I would need the boat’s interior volume, and to get that I would have to resort to some scientific sleuthwork. Over the course of a month, I downloaded every photo and diagram I could find of the Hunley and measured them meticulously to find all the sub’s relevant dimensions. After I used this information to create a three-dimensional model, my computer could tell me the size.

Based on the interior volume of the boat, I calculated how long it would take for painful levels of CO2 to build up, and determined the crew’s precise oxygen supply. The crew would have had a 30- to 60-minute window of warning—depending on their levels of physical exertion—between the time the air first reached a noticeable 5 percent CO2 and when it reached the low-oxygen level of 6.3 percent at which they might pass out. Carbon dioxide causes pain; the headache is sharp and profound, and the ragged panting feels like the body is struggling to catch up after a panicky sprint. It was implausible that the crew would have stayed peaceful and quiet for this length of time during such symptoms.
I had surpassed the threshold of reasonable scientific evidence, and therefore, for me, the theories of suffocation and asphyxiation were eliminated. Once I ruled out those theories, I turned back to examining my primary suspect: the blast.
* * *
The author Kurt Vonnegut once spoke in an interview about his time in the military in Germany during World War II, right after the firebombings that devastated Dresden. His job had been to excavate the bomb shelters and basements to remove the rotting corpses before the entire city started to stink of human putrefaction. The people he found had usually died without moving, without any signs of struggle, and were often still seated in their chairs. They were not outwardly wounded; they were not blown wildly across the room.
There are multiple ways for victims to die in a firebombing, and Vonnegut’s cases cannot be retroactively declared to have all occurred solely because of one single cause. However, they share the same key descriptors as the Hunley’s: undisturbed, no external injuries, dead where they sat or stood. To a blast researcher, this scenario sets off all the mental alarms. It starts our heads screaming that we should at least suspect what is called by our field a “primary blast injury.”
Medically speaking, the injuries from an explosion are neatly divided into one of four categories. A blast victim can receive only one type of injury, or they can receive a grab bag of trauma containing any mixture of the four. The injury types are numbered for easy reference: primary, secondary, tertiary and quaternary. The last three injury types are logical, meaning that they make obvious sense, and even people with zero blast experience can predict that they are expected possibilities.
In contrast, a primary blast injury—the kind possibly incurred by the victims in the Dresden bomb shelters—is a strange and horrifying fluke produced by the bizarre physics of an explosion. It is usually the result of a shock wave.

A shock wave is a particular kind of pressure wave, and it can have a terrible impact on certain human tissues. It most commonly develops during an explosion, when molecules of air accumulated at the wave front are shoved together by the explosive gas urgently expanding behind them. These molecules are so densely packed that they collide with one another far more rapidly than usual, generating a unique wave that moves faster than the normal speed of sound.
In its purest form, as defined by physics, the shock wave goes straight from zero to its maximum pressure in an instant; the change is so abrupt that, on a graph, it’s a vertical line. If it were a car it would go from 0 to 60 in 0 seconds. When the pressure of one of these waves reaches a certain threshold, it can disintegrate everything in its path. In blast physiology, we use the term a bit more loosely: Humans are so frail that we can die from fast-rising blast waves that don’t even qualify through physics as proper shock waves.
Most of the human body handles fast-rising waves surprisingly well. Such waves can move straight through water without causing much chaos and disruption, and human bodies are, after all, mostly water. It’s the gas pockets inside certain organs that cause the real drama. In the chest wall, which is mostly water, sound moves at roughly 1,540 meters per second. In the lungs, sound waves have to navigate a labyrinth of air bubbles, and they slow down to 30 meters per second. Therefore, a wave moving through the body that hits the lungs is suddenly forced to slow down by 98 percent.
If a shock wave traveling through the watery tissue of the chest wall is like an out-of-control semi-truck speeding down a mountain highway, then lung tissue is the gravel pit of a runaway truck ramp. The truck itself suddenly slows to less than 2 percent of its prior speed—but its great kinetic energy must still go somewhere. Cargo goes flying, gravel flies everywhere. Likewise, the delicate tissues that form the walls of the lungs rupture and shred, and blood sprays into the alveoli, the gas pockets needed for breathing. This breakdown is called spalling.
Brain tissue can also be affected by a shock wave, which can cause traumatic injury without ever damaging the skull. Critically, the brain remains intact after a primary blast injury, and the only potential sign of trauma is a faint inkblot of blood that may be spread across its surface.
Fatalities from a primary blast occur at lower pressures than the pressure levels required to translate a human body. To rephrase that in plain English: A person will die, choked with blood, from a shock wave that was far too weak to move him.
* * *
I needed to go beyond my theory and actually test my blast idea, which meant I needed a model submarine and a body of water. My labmates and I conducted preliminary experiments at Duke’s Chilled Water Plant 2, which hosts a picturesque reclaimed water pond. The results were encouraging, but we needed to scale up and also conduct the experiment with black powder. Duke’s facilities were not an option; Dale and I knew without even asking that the safety office would never allow live explosives on campus. My boyfriend, Nick, helped find a test site: an isolated, expansive tobacco, cotton and sweet potato farm with an artificial pond. The owner, Bert Pitt, asked me to drive out to talk before he agreed to the project. Understandably, he had some questions.
Sitting on barstools at his white kitchen counter, Bert and I looked at pictures of the Hunley on my laptop as I explained the project. I was using a scale model, I said, not a full-sized 40-foot sub, so while I didn’t plan to sink it, if something unexpected happened, the boat would be easy to retrieve. Bert was worried about the pond’s fish surviving the blasts. I told him that fish are surprisingly robust, because fish don’t have bubbly lungs that would halt the blast wave and tear apart. Unless they tried to eat the charge, they should be fine. Bert nodded, then gestured through the kitchen’s sliding door toward the silver pickup truck outside.

“Well,” he said, “let’s drive out there and see if the pond has got what you need.”
The pond was beautiful, both in the traditional, picturesque sense and also in terms of my scientific perspective. “It’s all yours if you think it’ll work for what you need,” Bert said, watching me sidelong as we stood on the wooden pier, looking out over the water. I tried to suppress my joy and instead just firmly shook his hand.
“It’s perfect. Thank you.”
* * *
Nick decided he was up for a lengthy drive to a mysterious munitions warehouse deep in the country. Brad Wojtylak, an agent with the Bureau of Alcohol, Tobacco, Firearms and Explosives, had called ahead so I could legally buy black powder in bulk. The warehouse was full of industrial shelving stocked to the brim with powder, ammunition, targets and security boxes aimed at helping doomsday preppers bury and hide their gold and bullets. We carefully lodged 20 pounds of freshly purchased black powder—the maximum amount permitted in one vehicle—in the trunk of my little Pontiac.
We were on the highway heading east when the car in front of us started spinning in erratic circles. I never saw what caused the accident. Something sparked the coupe two cars forward to hit the concrete barrier that divided our left-hand lane from westbound travelers. The coupe begun to turn doughnuts down the highway, catching the front end of the next vehicle in the line, metal and plastic and glass flying off like whirling shrapnel.
A moment before the chaos, I had noticed in the rearview mirror the grille of a massive truck pressed nearly up against us, and now my eyes were glued to the mirror despite the rapidly shrinking distance between us and the melee ahead. Nick had the same thought I did, and spoke only two words while digging his fingers into the handle of the passenger-side door. “BEHIND YOU.”
My brain shrieked: Black powder is impact-sensitive. We are a bomb.
I hit the brakes and we came to a heated stop several feet from the crash. The truck behind me was so close I could see the wide-eyed fear in the driver’s eyes in my rearview mirror.
He should have been far more terrified.
* * *
Several days later, I drove cautiously over the red dirt paths crisscrossing Pitt Farm. Crouching in the long grasses at the end of the pier, I tightened the small access panel that shielded the interior of our six-foot test submarine from splashing water. I had christened it the CSS Tiny, and stenciled the moniker onto its stern.
I’d been struggling with a problem: It wasn’t my first day at the pond, and throughout our testing, the gauges I was using would work fine when we tested them beforehand but failed inside the boat during the test. The readings still didn’t make sense. Some degree of pressure transmission through the hull was almost inevitable.
After one of these failures, I asked the undergrad helping me to hit the bow with a rubber mallet to help me test the gauge. Unfamiliar with nautical terminology, he brought the mallet down squarely on the stern instead. I stared at him for a moment, processing the realization that not everybody knew the difference between bow and stern.
Then I had my eureka moment.
I grabbed the mallet and smacked the bow hard. The pressure reading inside the boat jumped. I hit the stern. Nothing. I understood then why the internal gauges kept failing: They could only read pressure waves traveling from one direction. They were facing the bow and wouldn’t read pressures coming from any other direction.
I had assumed, because the charge was attached to the ship’s bow, that much of the pressure would naturally transmit from that direction. It turned out it was coming in from another direction, and I’d been missing it because I had pointed my gauges the wrong way.
Once I realized what was wrong, I borrowed a new set of underwater gauges from other Navy engineers—and these gauges were omnidirectional. That meant they could measure waves coming from any direction. The new gauges worked like magic. With each test, they showed an internal increase in pressure precisely with the arrival of the blast wave. This initial increase was followed by exactly what I expected: a jagged, erratic waveform of pressure, the initial wave bouncing around inside the small enclosed hull. The pressures were getting in, just not through the bow.
My research partner, Luke, a medical student and former Army explosive ordnance disposal operator, carried the first charge from his truck to the shore and attached a black powder charge to the bow of the model boat. The 283-gram charges, like the model itself, had been built to a carefully measured 1/6 size scale. As he pulled the Tiny into the center of the pond, long, black foam-insulated wires trailed out behind it.
I triple-checked the gauges’ signals on my screen and held up a hand to Brad, the benevolent ATF agent who had volunteered to help with our tests. He bellowed the countdown and pushed the button on the blast box to trigger. First, I saw the plume of the geyser of water. Then I felt the pier vibrate. Last of all, I heard the blast.
Brad yelled from shore that he could feel that charge through the ground. What he meant was: This one was strong. Stronger than any of our previous tests with the boat. I was too consumed by staring at the whirring laptop to respond in any meaningful way. I waited for the screen to display the pressure waves from the charge.
There it was, the data from the pressure gauge tracking across the monitor of my computer. The squiggly neon green line—plotting pressure versus time—showed the jagged, erratic scream of bouncing waves trapped inside the hull of the boat. It had had sharp peaks, peaks with rapid rises—peaks that weren’t technically shock waves but still rose to maximum in under the two-millisecond rise speed that would hurt human beings.
We set off as many charges as we could before the sun began to set on the pond. Blast after blast, we captured and saved the waveforms. I was thrilled to see that the readings looked consistent. And like the actual Hunley, the scale-model Tiny refused to show any damage itself, even after repeated blasts, even as it transmitted the pressures inside.
By the end of the day, the data saved on the laptop was worth more to me than anything I owned. I immediately backed it up in triplicate.
The next step was to translate all the squiggly pressure traces into a meaningful description of what happened on that cold night in February 1864. My end goal was not simply to sit in a series of muddy ponds and set off charges. It was to determine whether the crew had been killed by their own bomb while cocooned inside the steel walls of their vessel.
Scientists do not like to throw around the word “proof.” We couch our words carefully. So because I am a scientist, here is the fine-print scientific disclaimer: There are other possible ways to explain how this pressure got inside the vessel and maimed the crew. But the theory I was beginning to develop was the most likely candidate, given the data that I had.
My analysis showed that the amount of pressure ricocheting around inside the metal tube, combined with the quick rise time of the wave, would have put each member of the Hunley’s crew at a 95 percent risk of immediate, severe pulmonary trauma. The kind that would leave them gasping for air, possibly coughing up blood.

Researchers had studied the remains of the Hunley crewmen and found that some had apparently undamaged skulls and intact brains. The soft tissues were severely damaged and shrunk by long-term exposure to salt water, but medical personnel who carefully examined the tissues found that some of the brains bore diffuse stains consistent with blood.
* * *
The sailors in the Hunley would not have had time to realize the twinned truths of their victory and demise.
Inside the submarine that night, they all had items in their pockets that spoke of their belief that they would go on living. The smokers brought their pipes. George Dixon, in his 20s with a head full of blond hair, brought his pocket watch. The watch broke at the time of the attack, locking the hands forever at 8:23 p.m. Dixon’s head dropped against the side of the hull. His ankles were lightly crossed, and one hand fell to his thigh, his body propped up by the hull wall and his small captain’s bench.
The deck of the Housatonic had sprayed into a million shards of wood and metal hurtling into the air. Most of the crew had already run for the bow and safety, but as the ship gave a mighty heave to port, the few remaining joined in the mad dash forward. A cloud with the noxious stench of rotten eggs from the black powder drifted off across the smooth surface of the calming ocean. Five Union sailors had been killed.
The submarine drifted on the outgoing tide. With no one alive to operate the bilge pumps, eventually, it started to sink. Water rushed in, bringing the little boat to the sand but leaving an air space, inside of which, over the decades, stalactites would grow. The HL Hunley and its crew settled to a quiet grave 30 feet beneath the dark blue waves.
From In the Waves: My Quest to Solve The Mystery of A Civil War Submarine by Rachel Lance, to be published April 7 by Dutton, an imprint of the Penguin Publishing Group, a division of Penguin Random House, LLC. Copyright © 2020 by Rachel M. Lance
A Note to our Readers
Smithsonian magazine participates in affiliate link advertising programs. If you purchase an item through these links, we receive a commission.
:focal(3611x1874:3612x1875)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/bc/42/bc425e97-f508-44cd-a4e8-a1e031325c35/opener-mobile.jpg)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/e9/59/e959bb10-4715-4279-aa2c-0d82aec3496a/opener_updated.jpg)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/rachel_lance.png)


/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/rachel_lance.png)