Could This Bionic Vision System Help Restore Sight?
The technology gives hope that blind patients, who lost sight from disease, might one day emerge from the dark
:focal(4452x2083:4453x2084)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/04/96/049661cc-162a-4641-804a-5d06459daa7d/eye_ball.jpg)
Time is not your body's friend. Years will wear away the color of your hair, dull the bounciness of your joints, erase the elasticity of your skin. Among these many indignities of age, however, one of the worst is the potential loss of sight.
The leading cause of age-related vision loss is macular degeneration—a disease that slowly eats away at the central vision, leaving a blurry or dark hole in the middle of your field of view. The National Institutes of Health estimates that by 2020 nearly three million Americans over the age of 40 will suffer from some stage of the disease. But vision loss isn't restricted to the elderly. Retinitis pigmentosa, a genetically inherited disease, also strikes around 1 in 4,000 people in the United States—both young and old.
The diseases target the photoreceptors, which are the rod- and cone-shaped cells at the back of the eye. These cells convert light into an electrical signal that travels to the brain via the optic nerve. Macular degeneration and retinitis pigmentosa break down these photoreceptors. In the most advanced forms of the disease, many tasks become nearly impossible without assistance: reading text, watching TV, driving a car, even identifying faces.
Though the impacts are severe, not all hope is lost. The remainder of the retina's neurons and cells that transmit the electrical signals are often left intact. That means that if scientists can rig a device that can essentially imitate the function of the rods and cones, the body can still process the resulting signals.
Researchers and developers around the world are attempting to do just that. A team at Stanford is using a small and sleek solution: tiny photodiode implants, a fraction of the width of a hair across, that are inserted underneath the damaged part of the retina.
“It works like the solar panels on your roof, converting light into electric current,” Daniel Palanker, professor of ophthalmology at Stanford University, says in a press release about the work. “But instead of the current flowing to your refrigerator, it flows into your retina.”
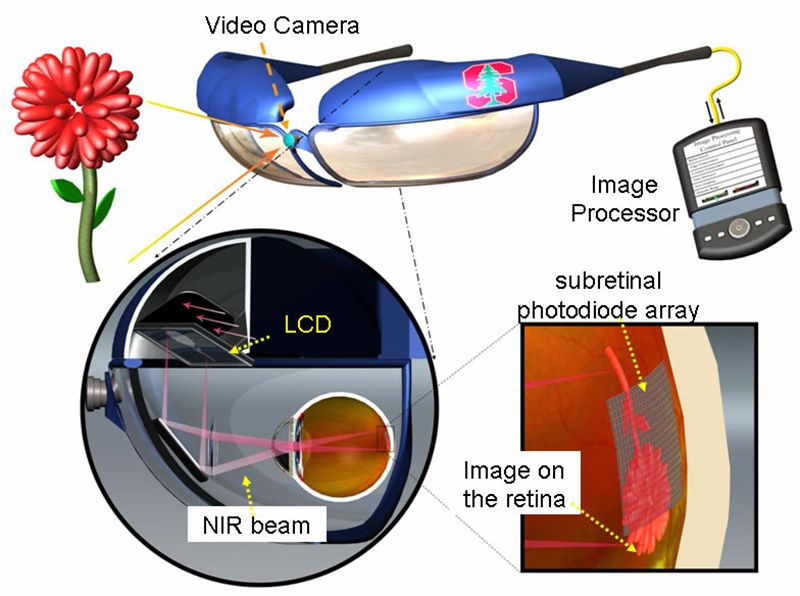
Dubbed PRIMA (Photovoltaic Retinal IMplAnt), the minute panels are paired with a set of glasses that have a video camera embedded in the center. The camera takes pictures of the surroundings and wirelessly transfers the images to a pocket computer for processing. Then the glasses beam the processed images to the eyes in the form of pulses of near infrared light.
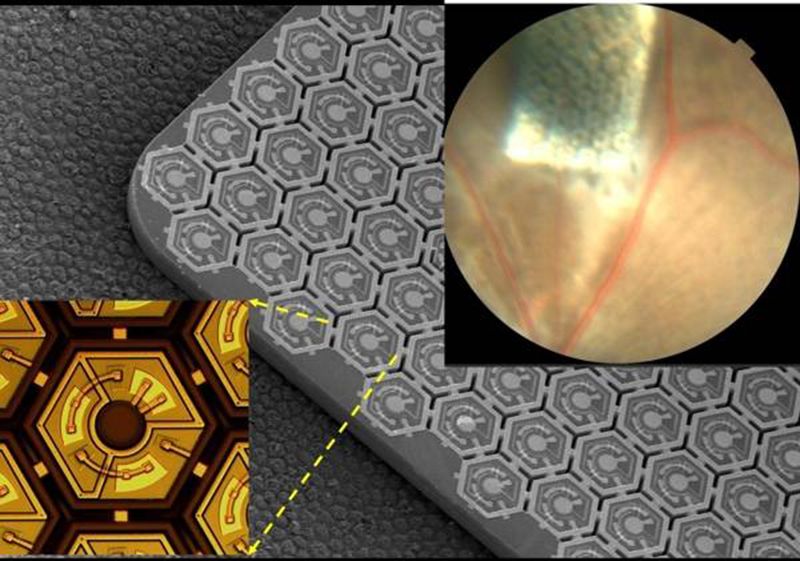
The tiny array of silicon "solar panel" implants—each roughly 40 and 55 microns across in PRIMA's latest iteration—picks up the IR light and converts it into an electrical signal, which is sent through the body's natural network of neurons and converted into an image in the brain.
To test out the device, the team implanted the tiny PRIMA panels in rats, then exposed them to flashes of light, measuring their response by electrodes implanted over the visual cortex—the part of the brain that processes imagery. Using the 70 micron implants they had developed at the time, the researchers found that the rats had around 20/250 vision—slightly above legal blindness in the U.S., which is 20/200 vision. This means that a person can see at 20 feet what a person with perfect vision can see at 250 feet, rendering most of their surroundings blurry.
"These measurements with 70 micron pixels confirmed our hopes that prosthetic visual acuity is limited by the pixel pitch [or the distance from the center of one pixel to the center of the next pixel]. This means that we can improve it by making pixels smaller," Palanker writes via email. They've already developed pixels three quarters the size. "We are now working on even smaller pixels," he writes.
PRIMA is, of course, not the only team chasing this goal. A device called Argus II from Second Sight, a California-based company, has already made it to market in the U.S. Approved in February 2013 by the Food and Drug Administration for patients with severe retinitis pigmentosa, the basic setup is similar to PRIMA. But instead of a solar panel, the implant is a grid of electrodes, which is attached to a pea-sized electronics case and internal antennae. A glasses camera takes an image that is processed by a small computer and then wirelessly transmitted to the implant, which fires electrical signals to create the image.
But there are several drawbacks to this system. The implant's electronics are bulky and the antennae can experience interference from home appliances or other antennae-reliant gadgets, such as cell phones. The device also has limited resolution, restoring vision to around 20/1,260 without additional image processing. Because of this limited resolution, the FDA only has approved its use in patients who are almost completely blind.
"The FDA doesn't want to run the risk of damaging the vision in an eye that already has some, because the amount of visual restoration is minimal," says William Freeman, director of the Jacobs Retina Center at the University of California San Diego. "You can get a little bit, but it's not a lot."
Many more technologies are also in the works. A German company Retinal Implant AG uses a digital chip, similar to what is found in a camera. But preliminary tests for the technology in humans have been mixed. Freeman is part of another company, Nanovision, which employs nanowire implants that are barely larger than a wavelength of light. Though they work similarly to PRIMA's photodiodes, Freeman says they have potential to be more sensitive to light and could help future patients see on a grayscale—not just black and white. The technology is still in animal trials to evaluate its effectiveness.
"[For] all these technologies, there are limitations that are intrinsic," says Grace L. Shen, director of the retinal diseases program at the National Eye Institute. Though not directly involved in prosthesis research, Shen serves as the program officer for one of the grants that supports Palanker's work.
PRIMA addresses some of the limits of electrode-based solutions like Second Sight. Though the images it produces are still black and white, PRIMA promises higher resolution without the need for wires or an antenna. And because the implants are modular, they can be tiled to suit each individual patient. "You can put as many as you need to cover a large visual field," Palanker says.
Prima is also easier to implant. A section of the retina is detached with the injection of fluid. Then a hollow needle loaded with the solar panels, essentially, is used to position the panels in the eye.
But as with all eye surgeries, there are risks, explains Jacque Duncan, ophthalmologist at University of California, San Francisco, who was not involved in the work. For the sub-retinal surgery PRIMA requires, these risks include retinal detachment, bleeding and scarring. There's also a possibility that if the device is not placed correctly, it could damage residual eyesight.
That said, Duncan’s take on the new device is positive. "I think this is an exciting development," she says. "The PRIMA approach holds a lot of potential to provide visual acuity that might be comparable to, or even better than, the currently approved Second Sight ARGUS II device."
As Anthony Andreotolla, a patient with an Argus II implant, told CBS earlier this year, his vision is certainly limited: "I can tell the difference between a car or a bus or a truck. I can't tell you what make the car is." But the prospect of further advances is giving patients—including Andreotolla, who suffers from retinitis pigmentosa and lost all vision by the time he reached his 30s—hope for the future.
PRIMA still has a long road ahead before it's ready for market. The team has partnered with Pixium Vision of France and together they are working towards commercialization. Palanker and his co-inventors hold two patents related to the technology. The next step is human trials, the first of which was just approved by the French regulatory agency. The trials will start small, just five patients who will be studied over the course of 36 months. "We want to see what the thresholds are and the surgical issues," says Palanker.
These tests will serve as the proving grounds for the device, says Shen. "Until they really test it in humans we couldn’t be sure what the benefits are."
Right now, Shen explains, the visual clarity the devices impart are not what she considers "meaningful visual images." That can only be achieved by a better understanding of the neural pathways. "If you just have a bunch of wires, it doesn't make a radio," she says. "You have to have the wiring correct."
The same is true of vision; it isn't a plug-and-play system. By mapping out the entire neural pathway, only then can researchers hope to create sharper images using prosthetic devices, perhaps even color images.
Palanker agrees. "Properly utilizing remaining retinal circuitry to generate retinal output as close to natural as possible should help improve prosthetic vision," he writes in an email.
There are also vision diseases where many of these solutions won't work, says Freeman. Vision loss from glaucoma is one example. "The inner retinal cells are dead, so whatever you stimulate there's no connections to the brain," he says.
But scores of researchers from all fields are on the case, pushing the boundaries of what we know is possible—engineers, material scientists, biologists and others. Though it may take a while, there's likely still more to come. Just like with our cell phones and cameras, says Shen, the systems have gotten faster, more efficient and smaller over the last couple decades. "I'm hopeful that we haven't reached our limit yet," she adds.
The key right now, Freeman says, is managing expectations. On the one hand, researchers are trying not to give people false hope. "On the other hand, you don't want to tell people this is a hopeless thing," he says. "We are trying, and I think eventually one or more of these approaches is going to work."
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/Wei-Haas_Maya_Headshot-v2.png)


/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/Wei-Haas_Maya_Headshot-v2.png)