Could This Head Gear Help Treat Parkinson’s Disease?
Students at Johns Hopkins University have created an at-home brain-stimulating device to ease Parkinson’s symptoms
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/07/84/07843368-e4ec-43eb-8f94-d10e0710bd36/stimband.jpg)
One million Americans suffer from the tremors, stiffness and slurred speech of Parkinson’s disease. Major depression affects some 16 million US adults a year, and nearly 30 million Americans deal with the pain of migraine headaches, while about 1 in 1,000 endure the agony of even more painful cluster headaches. Medications are usually the first-line treatment for these and other neurological conditions, with deep brain stimulation surgery—where a surgeon cracks a patient’s skull and places tiny electrodes in the brain tissue as a sort of “brain pacemaker”—sometimes used as a last resort.
What if, instead of side effect-ridden drug regimens or invasive surgeries, these conditions could be treated by painlessly stimulating the brain from outside the skull?
“What if there’s a way to do this noninvasively?” Ian Graham, a biomedical engineering graduate student at Johns Hopkins University, wondered after witnessing a multi-hour deep brain stimulation surgery for depression.
Transcranial stimulation, or stimulating the brain from outside the skull, has become one of the hottest areas in biomedical engineering. The method is usually done in one of two ways. One technique, called transcranial direct current stimulation, uses electrodes placed on the scalp to send electrical signals to the brain. The other, called transcranial magnetic stimulation, uses a magnetic coil on the scalp to produce electrical activity in the brain. Different locations of the brain are stimulated at different intensities and frequencies based on the condition being treated. While no one is sure precisely how brain stimulation improves Parkinson’s and other conditions, it’s understood that the stimulation can affect how neurons fire and can regulate neurotransmitters like serotonin and dopamine.
Graham and other biomedical engineers at Johns Hopkins invented a headpiece that uses electrodes to stimulate the brains of Parkinson’s patients. The STIMband device, which will begin clinical trials later this year or early next year, is meant to be used at home, which sets it apart from other transcranial stimulation devices. The students hope it will help deal with some of the more debilitating symptoms of Parkinson’s, including tremor and balance issues. Earlier this month, the STIMband won a $5,000 second-place prize in VentureWell’s BMEidea national design contest for biomedical and bioengineering students.

With STIMband, the students place the electrodes in locations known from computer modeling to stimulate parts of the brain affected by Parkinson’s. They observed patients participating in Johns Hopkins studies on transcranial direct current stimulation and were impressed by the results.
“I’ve seen a patient come in, and after treatment he had to sign his name,” says Graham. “He said he hadn’t been able to write like that in years.”
The students met with patients in the hospital’s Parkinson’s clinic over many months to gather data about what people really needed in an at-home device. Eventually, they came up with a battery-powered design roughly based on a baseball cap, which can be easily slipped on and controlled with a large button.
STIMband treatment would start in the neurologist's office, where the device would be fitted to the patient. The patient would then take the STIMband home and use it for 20 minutes a day, every day. Treatment might eventually be modified based on individual results, but Graham says the patients would likely use the STIMband indefinitely, as long as they're seeing positive results.
"Since PD [Parkinson's Disease] is degenerative, and the STIMband acts differently than the medication, it should also prove beneficial for a longer period of time," says Graham. "Unfortunately that period of time is still unknown."
If STIMband trials prove successful, the group hopes to achieve FDA approval. The device would likely cost between $600 and $1,000, depending on material choices.
Transcranial stimulation is currently being studied by researchers as a treatment for neurological and neuropsychiatric conditions, including epilepsy, stroke, Tourette’s syndrome, depression and mania, migraine, schizophrenia, eating disorders, dystonia (painful involuntary muscle contractions) and chronic pain. But the FDA has only approved transcranial magnetic stimulation for medication-resistant depression.
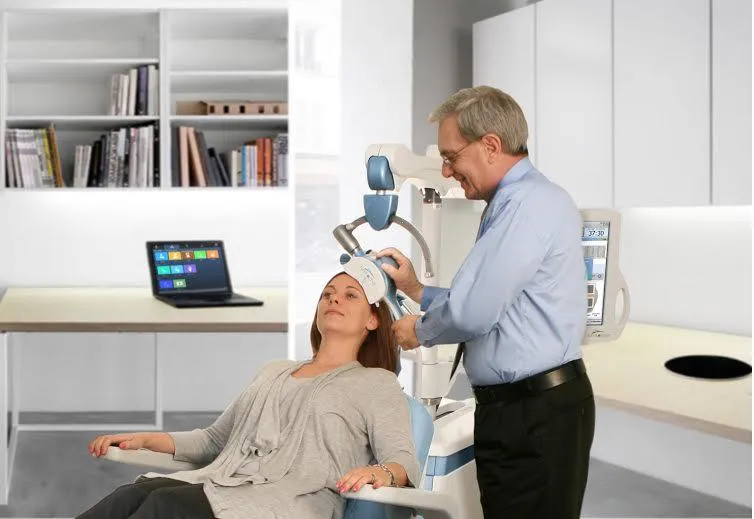
“This is not like placing refrigerator magnets on people’s heads,” says neurologist David Brock, the medical director of Neuronetics, the company that produces NeuroStar, a transcranial magnetic stimulation device for depression. NeuroStar treatment is given in a doctor’s office. For a period of four to six weeks, patients come in five days a week for 45-minute sessions. They sit in a chair reading or listening to music while the device, placed over the left side of their forehead, stimulates their left prefrontal cortex.
People often mistakenly consider transcranial stimulation to be an alternative treatment, Brock says, but it’s actually backed up by clinical data. Studies show about 30 to 40 percent of treatment-resistant depression patients go into remission after using NeuroStar, while more have some improvement of symptoms.
Nor is transcranial stimulation like electroconvulsive therapy (ECT), or “shock treatment,” the stigmatized but often highly effective depression treatment that uses electricity to induce a seizure. Unlike ECT, transcranial stimulation doesn’t induce a seizure and doesn’t necessitate general anesthesia or a hospital stay. It’s also free from ECT’s more notorious side effects, including memory loss and confusion.
Brock says transcranial stimulation will almost certainly become an approved treatment for other conditions in coming years, once researchers pin down the right location and intensity to treat the issue at hand. “[Transcranial stimulation] is a lot like a Swiss Army knife,” he says. “We’ve figured out how to use the blade, but we haven’t figured out how to use all the other tools yet.”
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/matchar.png)


/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/matchar.png)