Could a New Nanomaterial Reduce Greenhouse Gases?
Berkeley researchers have developed a way to split carbon dioxide into oxygen and carbon monoxide using a nano-mesh
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/ac/57/ac575887-732c-47a2-bd8c-ae08d3d2156a/smoke-stack-field.jpg)
Most methods of fighting climate change are about reducing greenhouse gas emissions: inventing cleaner power plants, engineering greener cars. Then, there’s the camp of researchers who focus on drawing gases from the atmosphere once they’ve already been released.
So-called “carbon dioxide capture” has been controversial, often dismissed as impractical or inadequate. Yet as global efforts to reduce emissions have proven difficult and sometimes disappointing, the approach seems increasingly alluring.
A new invention, from scientists at University of California, Berkeley, offers a novel take on carbon capture. The researchers have created a nanomaterial that destroys carbon dioxide by splitting it into oxygen and carbon monoxide.
Scientists have long tried to get rid of carbon dioxide by splitting its molecule. These splitting attempts can be energy-intensive, which defeats the environmental purpose. So reseachers have used various catalysts to speed up the reaction, reducing the amount of electricity needed to split the molecules. Many scientists have focused on porphyrins, ring-shaped organic molecules, to make these reactions happen. Though porphyrins can have various atoms at their centers, the ones used for this purpose are cobalt porphyrins, which are especially catalytically active. When these porphyrins are added to a solution with two electrodes, an electrolyte and some dissolved carbon dioxide, the porphyrins are attracted to the electrolyte. This causes the electrons to move to the carbon dioxide, splitting it into carbon monoxide and oxygen. But this approach has not been perfect. The porphyrins clump together and lose effectiveness over time, and the solutions used to make the process happen are environmentally questionable themselves.
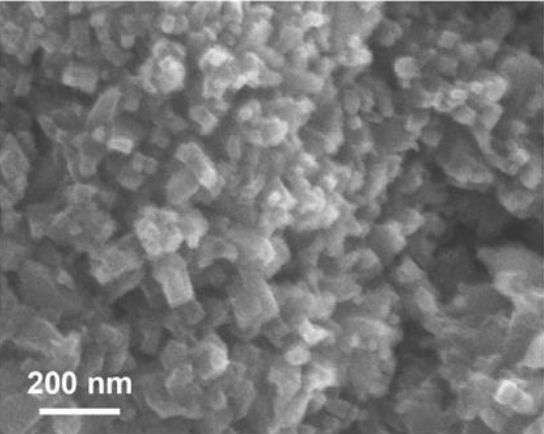
The Berkeley researchers seem to have found a new way to deal with this by creating a porous nanomaterial linking porphyrins together into a mesh-like substance. This is called a covalent organic framework (COF). The carbon dioxide percolates through the COF, splitting into carbon monoxide and oxygen with very little added energy. It works about 60 times more efficiently than splitting the carbon dioxide using free-floating porphyrins. The research was reported in the journal Science.
So what can be done with the oxygen and carbon monoxide created by the process?
“Carbon monoxide is important because it’s one of the feedstocks of the chemical industry, which makes fuels based on carbon monoxide,” says Christian Diercks, one of the lead researchers on the study. “The idea is basically to use carbon dioxide, which is a waste, and turn it into fuel.”
In the future, factories could use sheets of these nanomaterials around carbon dioxide-producing areas, such as smokestacks, turning it directly into carbon monoxide for fuel. But this is a long way down the road.
"If you really want to get something like carbon dioxide reduction to happen on a large scale, I think you always need government incentives," Diercks says, "because it always takes industry a long time to pick up new ideas like this."
So far, the lab has only made the material in tiny amounts, 30 milligrams at a time. It takes multiple days to produce, so the process will need to become more efficient to be implemented at an industrial level. The researchers' next step is to look into ways to more efficiently transform the carbon monoxide into fuel.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/matchar.png)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/matchar.png)