These Flexible Sensors Could Help Monitor a Stroke Patient In Recovery
Worn on the throat to evaluate speech, or on the body to track movement, stretchable sensors could lead to better rehabilitation
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/5f/cd/5fcd0431-a993-4781-b210-304bc4f06a19/throatsensor.jpg)
When stroke patients are discharged from a hospital or rehabilitation center, their recovery often slows or plateaus. Without regular intensive therapy and feedback, improving damaged speech and movement skills can be difficult.
A team of physicians and engineers at Northwestern University hope that a new device—a flexible sensor that can be stuck on the skin like a Band-Aid to monitor speech and movement—can help.
These sensors can be worn on the legs, arms and chest to detect motion, muscle activity and vital signs. The idea is that the devices could monitor patients’ movements while doing everyday activities and undergoing rehabilitation. Data would be streamed directly to doctors to monitor deficits and progress. It could help doctors see whether patients are continuing with their therapy, and which treatments are working, allowing them to suggest modified regimens if necessary.
The sensors, developed by John A. Rogers, an engineering professor who has worked on stretchable electronics for more than a decade, are currently being used in trials at the Shirley Ryan AbilityLab, a Northwestern-affiliated rehabilitation hospital.
“We’re able to look at somebody’s cardiac behavior, upper limbs, lower limbs, their ability to swallow, their sleep,” says Arun Jayaraman, a research scientist at the Shirley Ryan AbilityLab. “And we can continue to monitor them at home—is their balance good? Are they going to fall? Are both their legs moving in symmetry?”
One the newly developed sensors is a neck-mounted flexible electronic strip for monitoring speech.
“It’s almost like a digital wireless stethoscope, but one that can be placed directly on the throat,” says Rogers. “It picks up the vibratory signatures of speech. It’s actually measuring subtle vibrations in the skin of the throat region to monitor speech patterns, frequency of speech, cadence of speech.”
This device is particularly useful for patients with aphasia, the difficulties with speech common after strokes. Aphasias can range from the total inability to speak to mild difficulties in finding the correct word. Unlike microphones traditionally used by speech therapists to monitor speech, the devices can distinguish between the human voice and ambient noise, making monitoring in noisy everyday environments possible. The device can also monitor swallowing, which is a frequent problem in stroke patients. Poor swallowing can lead to choking, food aspiration and pneumonia.
“The key uniqueness here is that they’re soft,” Rogers says, of the sensors. “They can be mounted on any location of the body, even the neck, a very sensitive region.”
In fact, patients barely notice the sensors at all, Rogers says. One patient, when asked whether the neck sensor felt like a Band-Aid, said it was even less obtrusive.
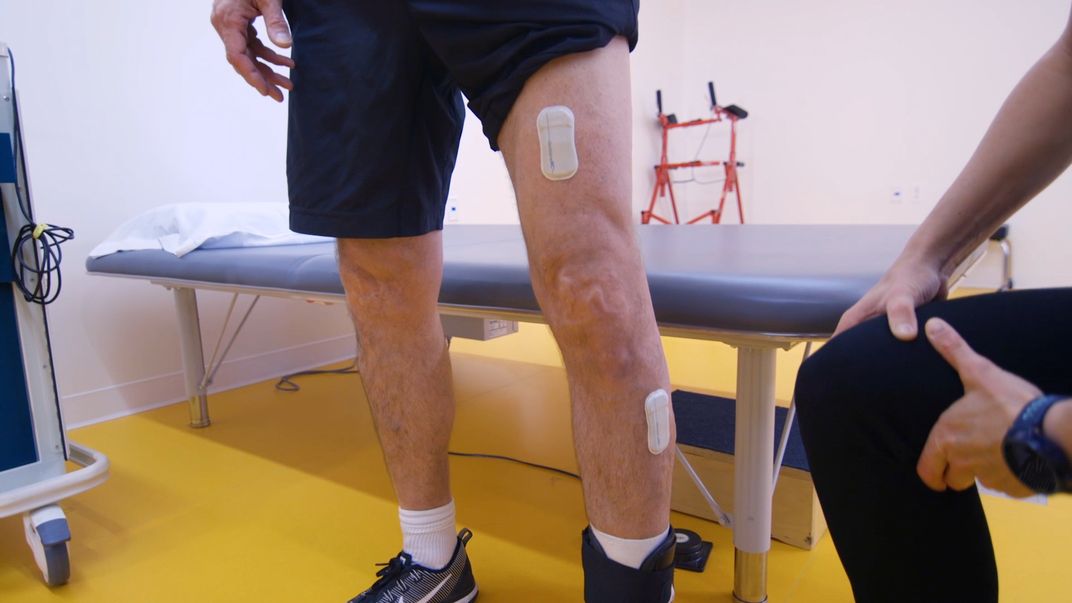
Normally, Jayaraman says, stroke patients’ progress is monitored at the doctor’s office, where they’re hooked up to wired sensors, a time-consuming, cumbersome process. Plus, since these visits only happen every so often—perhaps once a month—they can miss important patterns of gains and losses that could be key to unlocking the right rehabilitation regimen.
There's been a lot of recent interest in different wearable technologies to monitor patients' activities, says François Tremblay, a professor and physiotherapy expert at the University of Ottawa.
"But so far, these tools have not produced drastic changes in the way we deliver rehabilitation to patients," Tremblay says.
Sensors like those Rogers and his team have developed are interesting and potentially promising, Tremblay adds, "but the problem is that they can produce a vast amount of data and, most of the time, we don’t know how to interpret them in a meaningful way."
The team understands that making the data easily usable will be their next big challenge. Next steps will involve gathering much more data from both healthy and sick patients to make useful algorithms for monitoring progress. Data gathered from the sensor studies will need to be compared with data from traditional monitors, to make sure it’s reliable. The team also plans to develop an interface for doctors to receive and read the data on their phone or tablets. They hope to have a product widely available in the next several years.
The researchers also hope the sensors will be useful for problems beyond stroke—they are currently studying their potential for use on patients with Parkinson’s disease and spinal cord injuries. They’re also beginning to study whether the sensors could be used to detect cerebral palsy in high-risk newborn babies sooner than traditional methods.
“This has huge applications across the disease spectrum,” Jayaraman says.
The next big step could be to use the sensor in preventative medicine, tracking the movements and vital signs of healthy people and using an algorithm to detect whether they’re developing the early signs of Parkinson’s disease or are at risk of a health crisis like a heart attack.
“This can move science to a whole different field,” Jayaraman says.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/matchar.png)


/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/matchar.png)