In Pursuit of Better Baby Formula
Replicating human milk is no easy feat—nor is separating the science from the hype
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/a7/52/a752e723-2766-4cf6-8d38-308382438e2a/bottle_feeding_newborn.jpg)
Scan the aisles of any grocery store, and you’ll find a plethora of infant formula options, all designed to meet the nutrient needs of growing infants, who nearly triple their body weight in the first year of life. And yet researchers and companies are busy testing new formulations all the time.
That’s in part because much has changed in our understanding of breast milk’s complexities over the decades — from early knowledge of its nutrient composition to modern revelations that it’s a living, bioactive substance that evolved not just to nourish babies, but also protect them from pathogens, train their immune systems and send signals between mother and baby.
Formula may never be able to replicate all this complexity, but science could guide development of better products, says Tony Ryan, a neonatologist and emeritus professor at University College Cork in Ireland, who coauthored an overview of baby formula R&D in the 2019 Annual Review of Food Science and Technology. Though breastfeeding is optimal, “not every baby can be breastfed, and so we do need safe and effective formulas and with the maximum possible benefit,” Ryan says.
But it’s also a fact that companies are apt to hype the benefits of added ingredients. The “brain-nourishing” promises made for supplementing formula with the omega-3 fatty acid DHA, starting in the early 2000s, are a case in point. DHA increased the cost of formula, and it’s now ubiquitous across brands, but whether it’s necessary is controversial; a 2017 review of the scientific literature, published by the international research network Cochrane, found no clear evidence that it benefits babies’ brain development.
“As the understanding and the knowledge become more and more sophisticated, and we learn about new molecules and new things that are in breast milk, the goal would be to mimic that,” says Susan Baker, a pediatric gastroenterologist at the University at Buffalo Jacobs School of Medicine and Biomedical Sciences. But, she adds, ingredients should be added only if there’s evidence they’re beneficial, not just to sell more formula or increase its price.
So — how to separate the marketing from the science? Here’s a look at some formula ingredients under study, many of them already on store shelves.
First, a little history
Throughout time, alternatives to breastfeeding have always had their place, for example when mothers had to work, didn’t produce enough milk or died in childbirth. Until around 1900, wealthy families could hire a wet nurse, an arrangement that often compromised the health of the nurse’s own infant. Orphanages kept herds of lactating donkeys or goats, and babies would feed directly from their teats (which may have been safer than gambling with bacterial contamination of unpasteurized, unrefrigerated milk and hard-to-clean feeding vessels with nipples made from fabric or leather).
The emergence of formula, along with an understanding of germ theory, made feeding such infants simpler and safer. The earliest known patented formula was Justus von Liebig’s “soup for infants,” introduced in Germany in 1865 and made from cow’s milk, potassium bicarbonate and wheat and malt flour. Similar products followed, but most people used homemade recipes with affordable ingredients such as canned milk and Karo syrup, and supplemented babies’ diets with orange juice and cod-liver oil to prevent scurvy and rickets.

By the mid-1900s, as nutrition science advanced, formula companies were making better, more complex products, tweaking the types of protein and fat to better match human milk and supplementing with required vitamins and minerals. Today, parents who can’t or choose not to breastfeed can be assured that commercial formulas, governed by the nutrition and food safety requirements of the US Food and Drug Administration, are safe and meet a baby’s nutrient needs.
But there are detectable differences: Formula-fed babies are more likely to have gastrointestinal, respiratory and ear infections in early life, for example. Researchers and formula companies are still probing the suite of human milk molecules for new formula ingredients that might benefit babies’ health.
Prebiotics and probiotics
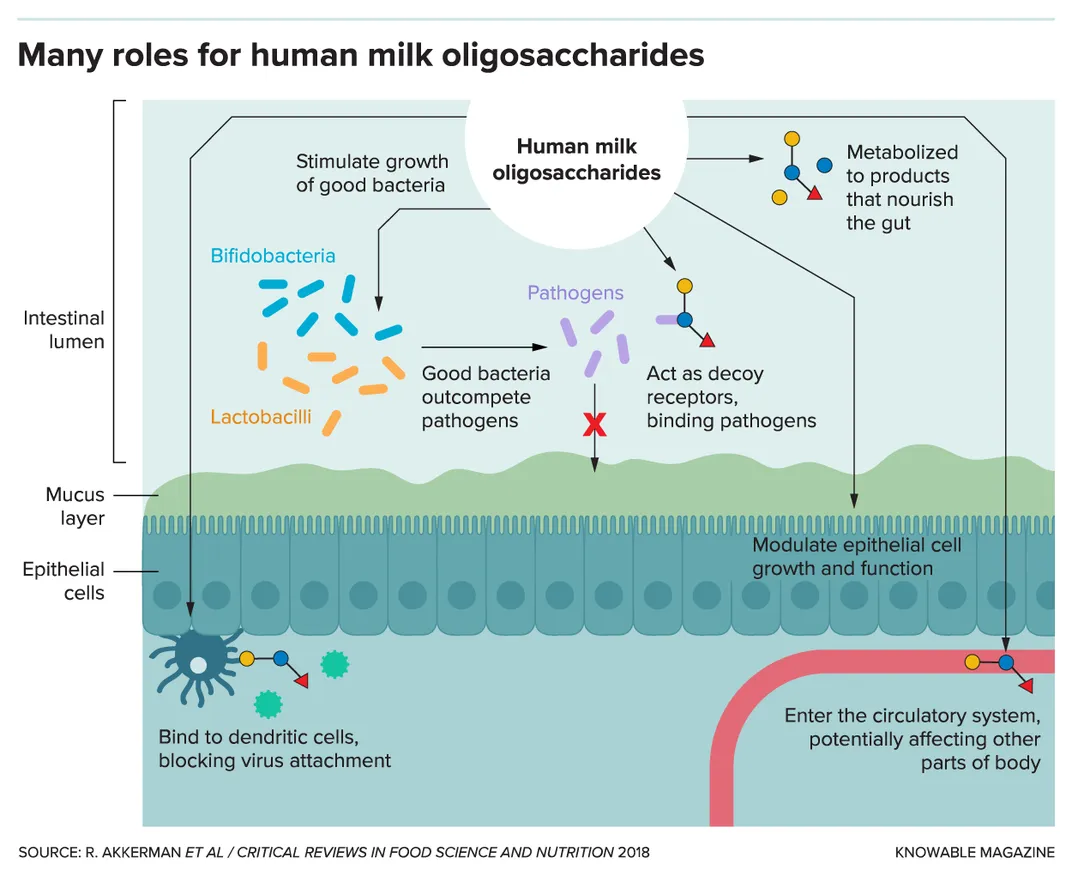
The third-most abundant component in human milk, after lactose and fat, is a large family of as many as 200 different sugar molecules called human milk oligosaccharides. Despite their prominence, they aren’t digestible by infants but instead serve as a food source for species of beneficial Bifidobacteria that dominate the gut microbiomes of breastfed babies, thus serving as prebiotics. The oligosaccharides also appear to act as decoys that can bind microbial pathogens and may prevent them from infecting the infant, and other antimicrobial and immune-modulating functions are being investigated by researchers.
As studies uncovered the importance of human milk oligosaccharides, so began attempts to mimic them in infant formula. But cow’s milk contains only a fraction of the oligosaccharides in human milk, and until recently the technology to synthesize large amounts didn’t exist. And so formula manufacturers instead added different, easier-to-make indigestible carbohydrates such as galacto-oligosaccharides and fructo-oligosaccharides, which also act as prebiotics for Bifidobacteria species.
But these molecules are structurally very different from human milk oligosaccharides and are unlikely to recapitulate their diverse functions, says Lars Bode, a nutrition scientist at the University of California, San Diego. “I’m always a bit skeptical when something is added to infant formula that is not inherently in human milk,” he says, “because you never know what these things do, really.” Bode points to rare reports of severe allergic reactions in children and adults from galacto-oligosaccharides and the fact that, overall, there’s little evidence that these prebiotics are beneficial. A 2018 review of 41 randomized controlled trials of prebiotic-supplemented formula concluded that while the products seemed safe, they didn’t lead to tangible health benefits.
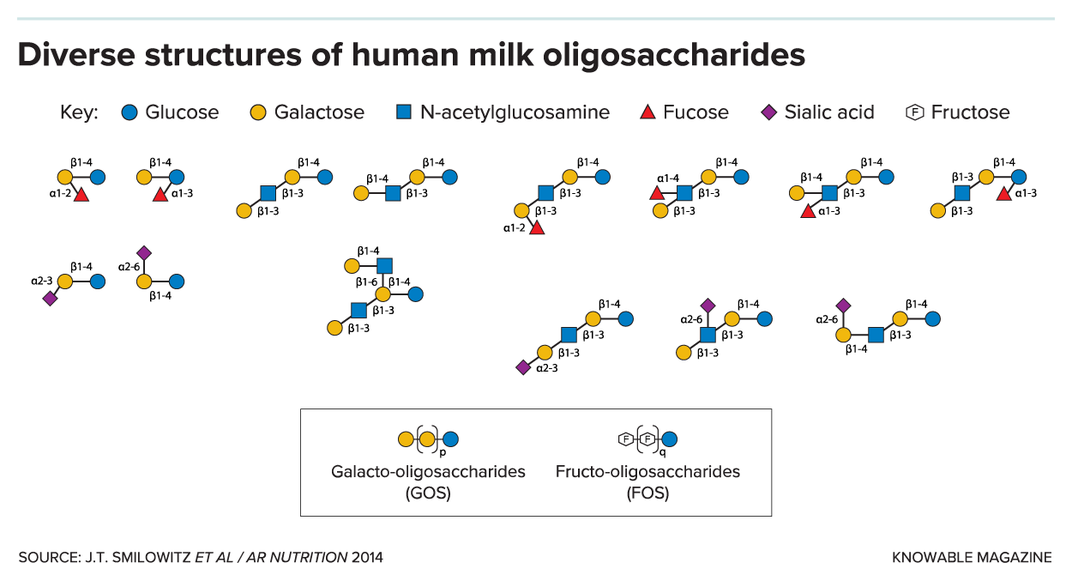
Several human milk oligosaccharides are now commercially available, their synthesis in bulk made possible by genetic engineering of yeast and bacteria. In a Nestlé-funded trial of a formula containing two of these, 2’-fucosyllactose and lacto-N-neotetraose, babies receiving the substances had a lower rate of bronchitis than babies receiving unsupplemented formula (10 percent vs. 28 percent), as well as lower rates of lower respiratory tract infections (19 percent vs. 35 percent) and antibiotic use (42 percent vs. 61 percent) in the first year of life, although the authors say these potential benefits need to be confirmed in larger studies.
Bode says this is a step in the right direction but that formula makers need to look beyond one or two oligosaccharides and also consider the importance of balance. “If you only give one oligosaccharide and if you start doing that in higher doses, you might get some effects that would otherwise be kept in check by adding other oligosaccharides as well,” he says.
In 2018, for example, he and colleagues reported that higher levels of 2’-fucosyllactose, lacto-N-tetraose and a third oligosaccharide in breast milk of mothers in India were associated with a greater incidence of symptomatic rotavirus infections in their babies, and that in cell culture experiments, the oligosaccharides increased the infectivity of a virus strain that causes severe gastrointestinal infections in infants.
Other studies suggest that specific oligosaccharides or mixtures of them in breast milk correlate with excessive weight gain and risk of allergies in breastfeeding infants. There could be potential in designing mixtures of five or 10 oligosaccharides that would benefit infant health, but more research is needed to identify which molecules to pick, and in what ratios.
Studies also have investigated adding different strains of bacteria, or probiotics, directly to formula. And here, too, results have been mixed, with some strains appearing to lower rates of diarrhea, and others leading to softer stools, but most showing no measurable benefit. “We’re on a very exciting pathway,” Ryan says — but with much more work still to do.
Lactoferrin
Lactoferrin is a protein found in high concentrations in human milk. It fights pathogens by binding to the iron they need to grow, and punches holes in the membranes of some bacteria. Lactoferrin concentrations are much higher in human milk than cow’s milk, and appear to rise in mother’s milk when the baby gets sick.
A couple of studies find benefits of adding lactoferrin to formula: One in China reported a decrease in the incidence of respiratory and diarrhea-related illnesses by 32 percent and 35 percent, respectively, and a small US study reported 70 percent fewer lower-respiratory tract infections. But the largest published study, conducted by Enfamil and enrolling 480 US infants, found that while lactoferrin-supplemented formula was safe and well-tolerated, it didn’t decrease infections or allergy symptoms. Even so, Enfamil now includes lactoferrin as an “immune-supporting protein” in one of its most expensive products.
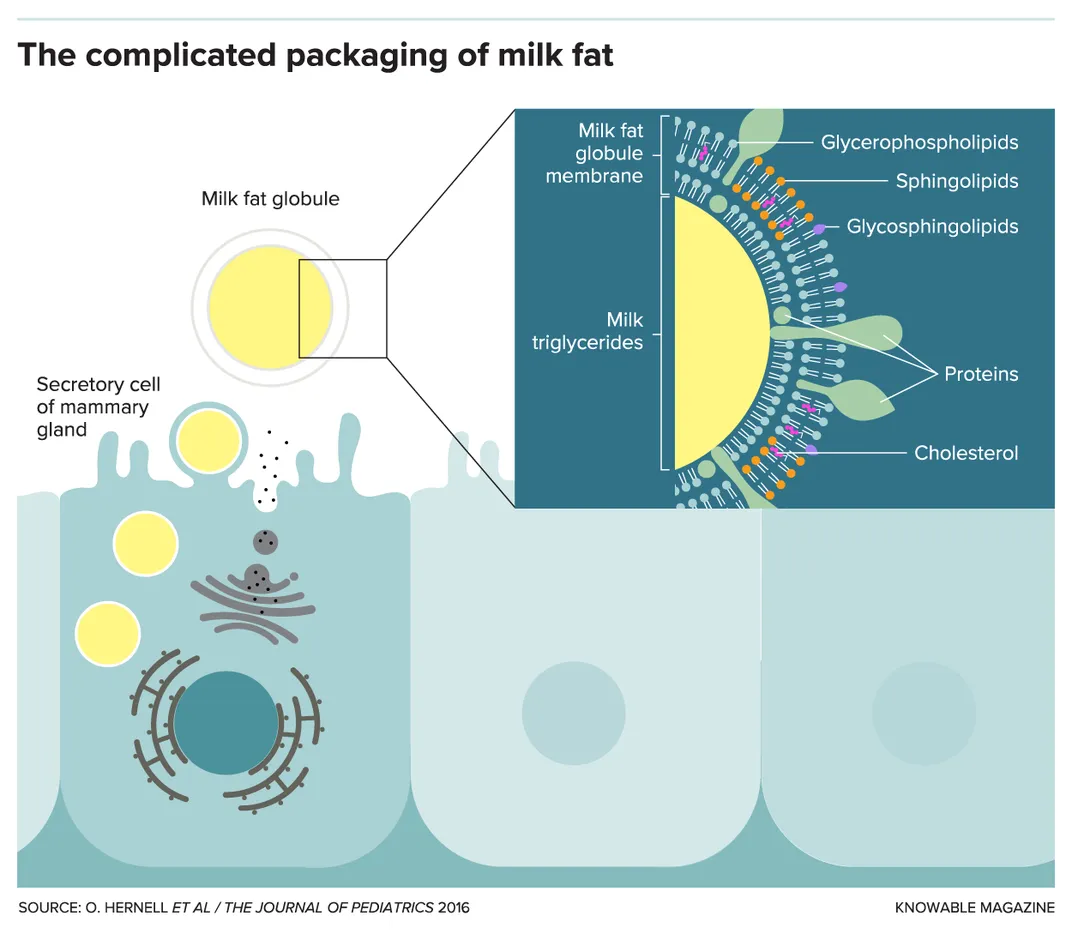
Milk fat globule membranes
When milk fat is secreted from the mammary gland, it’s packaged in a triple-layer membrane made of phospholipids, cholesterol and a multitude of proteins (including lactoferrin). Synthesis of these milk fat globule membranes is orchestrated by one of the most well-conserved parts of the mammalian lactation genome, says food scientist Bruce German of the University of California, Davis. Yet the membranes are discarded during manufacture of infant formula, which is based on nonfat milk powder with vegetable oils added as a fat source. “Evolution thought it was real important,” German says of the milk fat globule material. “Then we just threw it away.”
Researchers are now experimenting with adding the bovine version of milk fat globule membranes — often made from byproducts of dairy processing, such as butter- or cheese-making — to infant formula. This is probably a good idea, German says, but chronic underfunding of basic lactation research means there’s very little known about the role of the membranes in human milk, so it’s hard to know how to measure the effects of this addition. “Embarrassingly, we don’t even know the composition, much less the mechanistic function,” he says.
Trials of formula supplemented with bovine milk fat globule membranes have shown confusing results. One study, conducted in France and Italy and funded by Nestlé, found that babies grew normally and tolerated the ingredient, but they were no less likely to get sick. And there was a concerning outcome: Babies consuming one of the two experimental formulas were four times more likely to have eczema (13.9 percent vs. 3.5 percent in the standard formula group) — inflamed, itchy skin that often precedes the development of food allergies, hay fever and asthma.
But a Swedish study testing the same ingredient in a different formula recipe found no such effect. Funded in part by Swedish formula manufacturer Semper, it found that babies consuming the formula had fewer ear infections (1 percent vs. 9 percent in the standard formula group) in the first 6 months of life. And at 12 months, babies getting the supplement tested 4 points higher on a cognitive scale than those receiving standard formula, and the same as a breastfed group. Formula with milk fat globule membranes is now marketed in the US with the claim that it “supports cognitive development similar to breast milk."
Steven Abrams, a neonatologist at Dell Medical School at the University of Texas at Austin and chair of the American Academy of Pediatrics’ Committee on Nutrition, cautions against getting excited about these results. The cognitive scale used in the Swedish study, called the Bayley Scales of Infant and Toddler Development, wasn’t designed to measure small differences among a group of normally developing infants, he says, and “you can’t determine from a tiny difference on a Bayley at 12 months whether or not that child will actually be more likely to make it in to MIT.”
How solid are the findings?
Despite the potential for advances in infant formula and the claims of benefits made for these new formula ingredients, a skeptical eye is in order, researchers and clinicians say. Abrams, for his part, is not convinced that these new ingredients have been adequately studied, especially over the long term. Most studies in this area are funded by the formula industry, he adds, raising concerns of bias and making the case for more federal funding of infant nutrition research.
In 2015, Abrams published a commentary in the Journal of Pediatrics suggesting a moratorium on new formula ingredients until more research could be conducted. He notes that the Food and Drug Administration requires little clinical data on effectiveness or long-term safety before allowing addition of new ingredients. Since then, “the issue has gotten bigger, not smaller,” he says — with more new ingredients accompanied by vague, “structure/function” claims, such as “immune-supporting” and “brain-building,” based on minimal evidence. The FDA drafted guidance in 2016 that would require companies to show more meaningful clinical outcomes before making such claims, but the new guidelines haven’t yet been finalized and an agency spokesperson was unable to provide an estimate for completion.
Helen Hughes, a pediatrician at Johns Hopkins School of Medicine, says she doesn’t usually recommend any formula product over another, including those touting ingredients that mimic bioactive molecules in breast milk. A coauthor of a 2017 commentary in JAMA Pediatrics urging a higher bar for evidence for claims on formula labels, she worries that the claims may persuade parents to unnecessarily switch formulas or choose more expensive products — premium formulas with the newest ingredients can cost more than 50 percent more than standard products from the same companies.
“I, as a physician, would love to see more evidence about what they do before they’re added into formula,” Hughes says. “It’s hard as a parent,” she adds, “to say ‘I’m going to buy the formula that’s not for brain health.’ "
