Scientists Are Racing to Develop Paper-Based Tests for Covid-19
Inexpensive—and potentially at-home—tools could take only minutes to tell if someone is infected
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/a4/b5/a4b52e04-b243-4757-9dc3-8687be161e91/drive-in_covid_testing_site_in_miami.jpg)
Across the United States, there is a crippling surge in demand for coronavirus tests. In North Carolina, test results took an average of six to seven days in July, double from the previous month. In the District of Columbia, some residents waited more than 14 days for results, rendering the tests essentially useless as tools to tell people to self-quarantine and help break the chain of infection.
The U.S. is now running somewhere between 600,000 and 800,000 tests a day, according to the Covid-19 Tracking Project, a coronavirus data-gathering and reporting initiative. That’s an improvement over the roughly 150,000 daily tests run in April but still far short of the tens of millions of daily tests that, according to one report, are “critical to our ability to go outside again.”
“Our testing capacity, in my opinion, does not come anywhere close to our testing needs,” says Kevin Nichols, a diagnostics researcher at Global Health Labs, a nonprofit in Bellevue, Washington. And the scaling that’s needed is not likely to be achieved using current coronavirus tests, which require special equipment and expertise and can hardly keep up with demand as it is.
To reach the staggering amount of testing required to safely reopen the U.S., experts like Nichols say that our best bet is rapid, point-of-care diagnostic tests. Most likely, he says, ones made of paper.
Dozens of academic research groups and companies are racing to bring tests to the market that can rapidly detect SARS-CoV-2, the virus that causes Covid-19. Several of them use paper strips, borrowing a tried-and-true technology used for years in over-the-counter diagnostics such as pregnancy tests. These tests promise to be relatively cheap — perhaps under $10 each — and run without complicated instruments, meaning that they could even be used at home.
Early data suggest that these tests may not offer the nearly 100-percent accuracy of the currently used molecular tests. But the trade-off may be worth it: The ease and low cost of paper-based tests could help people return to some pre-pandemic activities with lower risk, Nichols says. “You buy a kit at the pharmacy, you test yourself and you know whether you can go see your grandparents this weekend.”
Testing: One, two, three
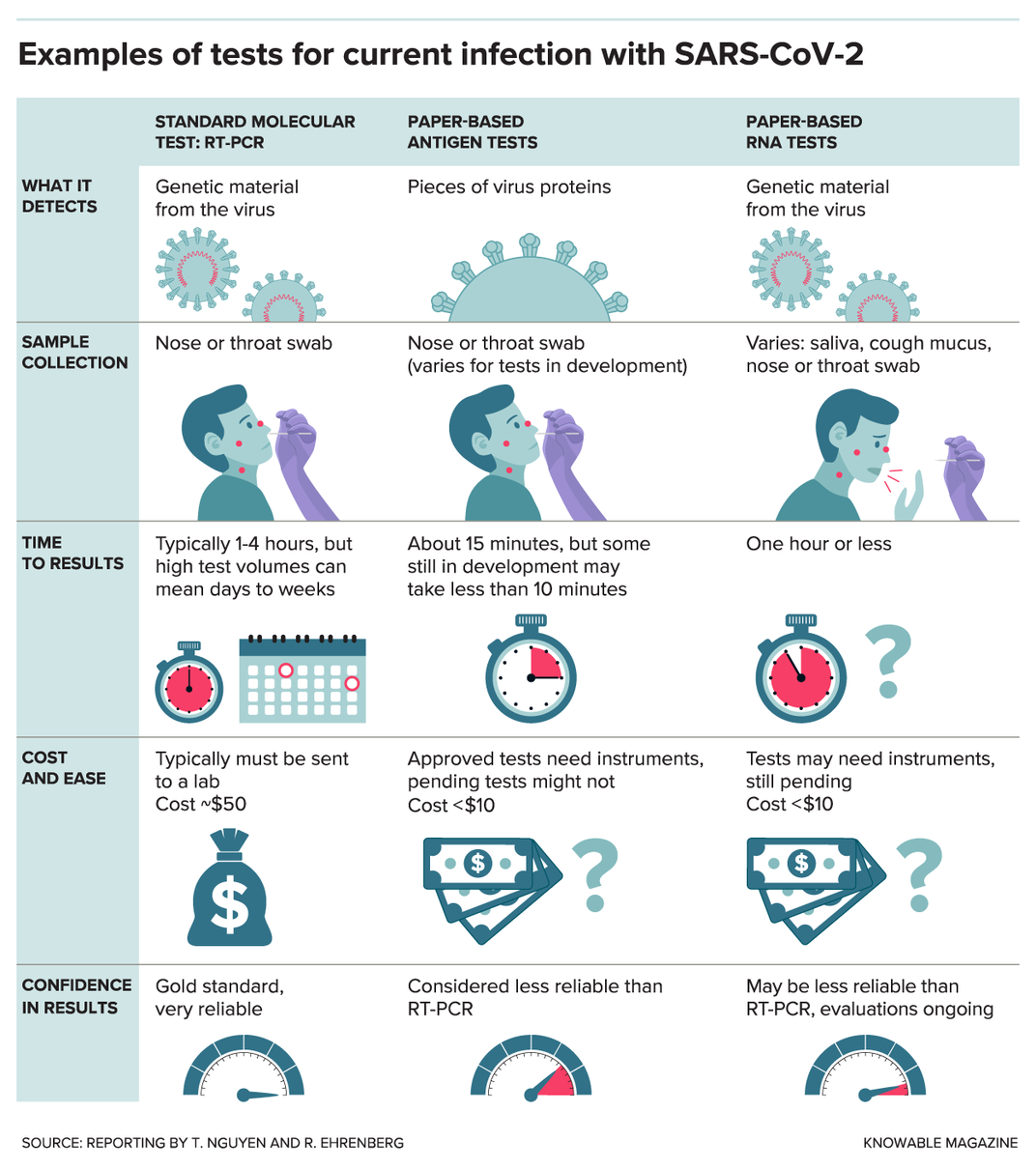
If you were to get a coronavirus test right now, it would most likely be an RT-PCR test (reverse transcription polymerase chain reaction); this test looks for sections of the virus’ genes. First, a swab from your nose or throat is sent to a lab. There, with the help of various chemicals and equipment, a molecular probe finds even a tiny amount of viral RNA and makes a DNA copy of it. A machine then produces millions of copies of this DNA and adds fluorescent tags, making it detectable by the device.
The RT-PCR test takes a few hours or less but the wait for results is usually at least a day — or even longer when labs are swamped or short on necessary chemicals. Yet once RT-PCR test results do arrive, they are very reliable, in large part because of the amplification step, which allows even trace amounts of the virus’ RNA to be detected.
Many of the paper-based tests in development take a different approach: They seek out proteins made by the virus, called antigens. These antigen tests typically use a technique called a “lateral flow assay” and work much like at-home pregnancy tests.
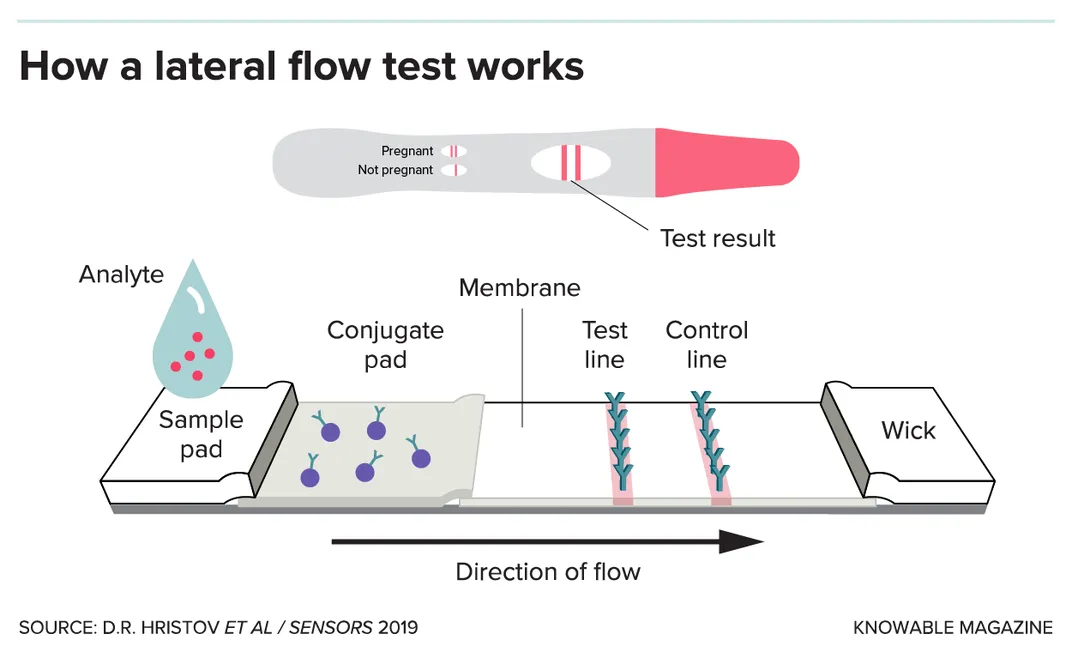
The tests use a paper strip typically coated with immune-system molecules called antibodies; in the case of a SARS-CoV-2 test, the antibodies recognize specific bits of viral proteins. The person’s sample is mixed with a small amount of liquid, which is applied to one end of the strip and then flows, via good old capillary action, toward the other end. Along the way, the sample passes through the antibodies (or similar binding proteins), which are snatched up by any viral antigens in the sample. This antigen-antibody combo migrates to the strip’s test zone and triggers a chemical reaction that causes a color change, indicating a positive result. Excess antibodies will surf the length of the strip to the control zone, and again cause a color change. That second change provides reassurance that the test is working as it should.
So far, two paper-based antigen tests have received emergency-use approval in the U.S.: the Veritor System by Becton, Dickinson and Co., and a test designed to run on a device called Sofia, manufactured by Quidel Corp. Both use instruments to read the results, and the Sofia test also requires that the testing lab has special certification. The tests give results within about 15 minutes.
Researchers are also getting closer to antigen tests that are simple enough for anyone to use at home.
One such test is being developed in the lab of Hadley Sikes, a chemical engineer at MIT. Her paper-based antigen test gives results within 10 minutes and doesn’t require a special type of membrane made of nitrocellulose to anchor antibodies onto the paper strip. This cuts out a manufacturing step. Instead, the test uses specially designed proteins that are bound directly to the paper to detect SARS-CoV-2 antigens.
Charles Henry, an analytical chemist at Colorado State University who coauthored an overview of paper-based analytical devices in a recent Annual Review of Analytical Chemistry, is working on several types of paper-based Covid-19 tests.
Two of his lab’s tests adapt a technique known as enzyme-linked immunosorbent assay (ELISA), which uses enzymes — types of proteins — to detect antigens. This approach usually involves several steps, but the team has condensed them into an almost all-in-one device, he says. (Henry plans to patent the design, so he declined to share many details.) To read the results, the team is working on two approaches: a visual signal and another method similar to a handheld glucometer used by diabetes patients.
Nichols’ lab, meanwhile, is advising the start-up company Luminostics, who has partnered with the pharmaceutical company Sanofi on another antigen- and paper-based test. Luminostics specializes in phosphorescent materials that glow in the dark, and the hope is that the test results could be easily viewed at home using just a smartphone and an attachment that blocks out light.
Although many of the tests in development use established technologies — lateral flow assays have been around since the 1970s, for example — adapting them for a new use and scaling up manufacture is no small feat. “Covid-19 has shown us that, yeah we have those technologies, but it’s really hard to develop new tests on a quick timeline,” Sikes says. “If you suddenly want 100 million of them, it’s hard to make that many all at once.”

A sensitive situation
A potential drawback of antigen tests is that viral antigens are harder to detect because proteins can’t be amplified the way that genetic material can. This is particularly a problem at the beginning of an infection when a person may not carry many virus particles.
But antigen tests can still provide actionable information — for example, should you go to work or not? — that’s more useful than waiting two weeks for results. With cheap, rapid tests, we could rethink our approach to testing, Sikes says. Someone could double- or even triple-check their test results over several days. That’s useful, because data suggest that false positives (testing positive when you’re not infected) are pretty rare with coronavirus tests, but there has been concern about false negatives (testing negative when you’re actually infected). These rapid tests could also help reveal infections in people who are asymptomatic. And people could always follow up a rapid test result with the standard RT-PCR test.
“The tradeoff,” Nichols says of an antigen-based test, “is that it’s not quite as sensitive but oftentimes it can be good enough to be useful.”
Researchers are devising various tricks to make their antigen tests sensitive enough to be practical. Nichols’ lab, for example, is screening thousands of antibodies in search of ones that are especially good at binding to the virus’ nucleocapsid protein, one of the most abundant viral proteins. That could up the test’s sensitivity. In July, the team published some of their results in advance of formal peer review, on the preprint site ChemRxiv.
Other labs are dealing with the sensitivity issue by developing paper-based tests that look for genetic material, but in a more straightforward manner than the standard RT-PCR tests. Some of these paper-based RNA tests use a method that amplifies viral material more quickly or requires heating the sample to only one temperature instead of the multiple rounds of heating and cooling needed for RT-PCR tests.
None of the paper-based RNA tests have been approved by the Food and Drug Administration yet. Clinical evaluations will measure, among other things, the tests’ reliability.
It’s tricky to tell how accurate these new tests are. Often, what’s reported is “sensitivity” — in medical testing parlance, sensitivity refers to “true positives,” meaning how often the test flags someone who really does have the virus. But sensitivity is just part of the equation.
There’s also test specificity, which refers to “true negatives,” meaning how often the test correctly rules out someone who doesn’t have the virus. On top of that, assessing test reliability depends on the testing population. For example, it’s easier to detect the infection in very sick people who have huge quantities of the virus than it is in people who have just been infected and don’t have many virus particles yet.
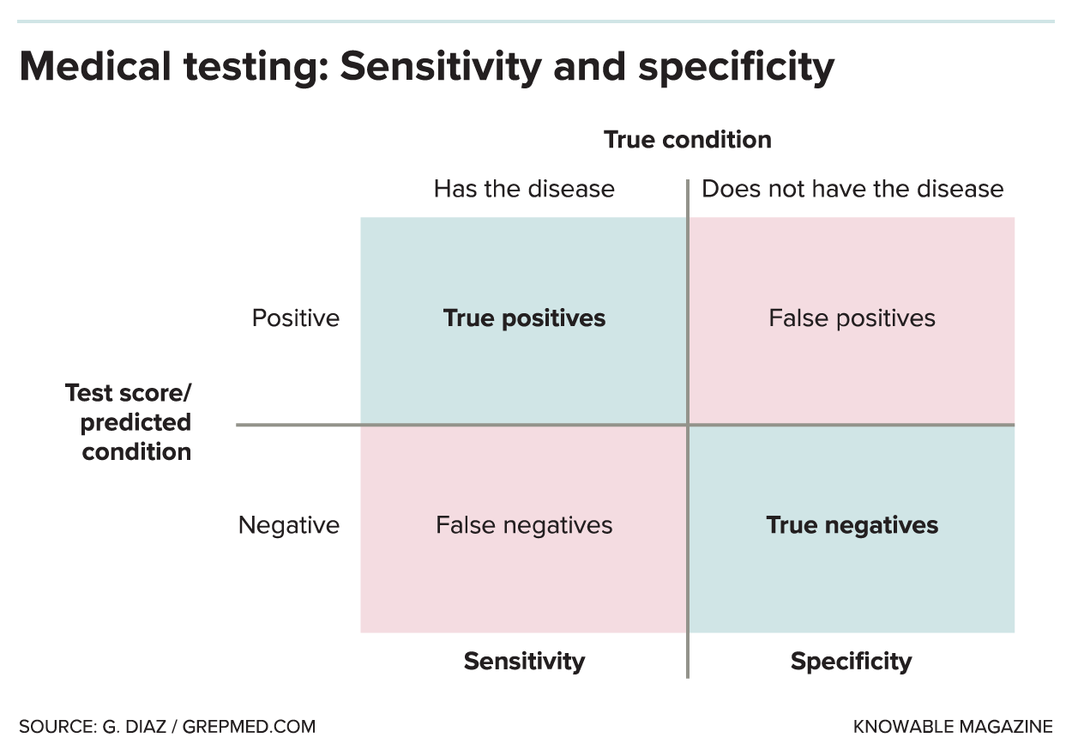
In the U.S., FDA guidelines direct test-makers to demonstrate adequate performance on a minimum of 30 positive specimens and 30 negative specimens. “That’s really, really subject to noise,” Nichols says, and makes a test’s accuracy hard to discern.
The paper-based tests that look for RNA should be more sensitive than antigen tests, but real-world findings of most of the still-unapproved paper tests remain to be seen. Nichols says he expects that regulatory requirements for tests will grow stricter in the coming months, which means that later tests will have a higher bar to clear.
The good news is that Henry predicts that at some point there will be clear winners that rise above their competition. “It’s really uncharted territory because never before have there been so many different tests developed all for the same thing,” he says.
Quality aside, distribution issues could also plague new SARS-CoV-2 antigen tests. In July, the Trump administration announced a one-time distribution of the two approved antigen tests for use in nursing homes in coronavirus hotspots. These tests could help nursing homes regularly test residents as well as staff, but there have already been concerns about shortages.
Sikes’ project, which is being developed in partnership with the manufacturer 3M, is one of more than two dozen selected by a National Institutes of Health initiative that aims to expand U.S. diagnostic testing capability to about 6 million tests per day by December. But FDA approval, manufacturing capabilities and other issues still need to be sorted out for that to transpire.
For now, researchers like Henry and the others are working as fast as they can to push their tests forward. “The running joke on a call yesterday was, ‘I’ll sleep sometime in 2022,’” he says. “At the same time, it’s exciting to think that we can do something that helps in some way — that’s the endgame here.”
