Soon, Your Doctor Could Print a Human Organ on Demand
At a laboratory in North Carolina, scientists are working furiously to create a future in which replacement organs come from a machine
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/09/43/0943f676-f58a-450d-9a71-9dfa30bd961d/may2015_a07_bioengineeredorgans.jpg)
On the second floor of the Wake Forest Institute for Regenerative Medicine, not far from the elevator bank, is a collection of faded prints depicting great moments in medical history. In one, an ancient Babylonian pharmacist holds aloft a vial of medicine. Another shows the Greek physician Hippocrates tending to a patient in the fifth century B.C. The prints were doled out to doctors half a century ago by the pharmaceutical company Parke-Davis, which touted them as a historical highlight reel. But it’s not hard to read their presence at Wake Forest, home to perhaps the largest concentration of medical futurists on the planet, as the ultimate in-joke: Can you believe how far we’ve come?
When I visited the institute, in the old North Carolina tobacco town of Winston-Salem, I passed airy laboratories where white-coated staffers glided back and forth across a tiled floor. On one table, arranged as if for an art exhibit, lay spidery casts of kidney veins, rendered in hues of violet and indigo and cotton candy. Down the hall a machine zapped sporadic electric currents through two sets of muscle tendons, one cut from a rat, the other engineered from biomaterials and cells.
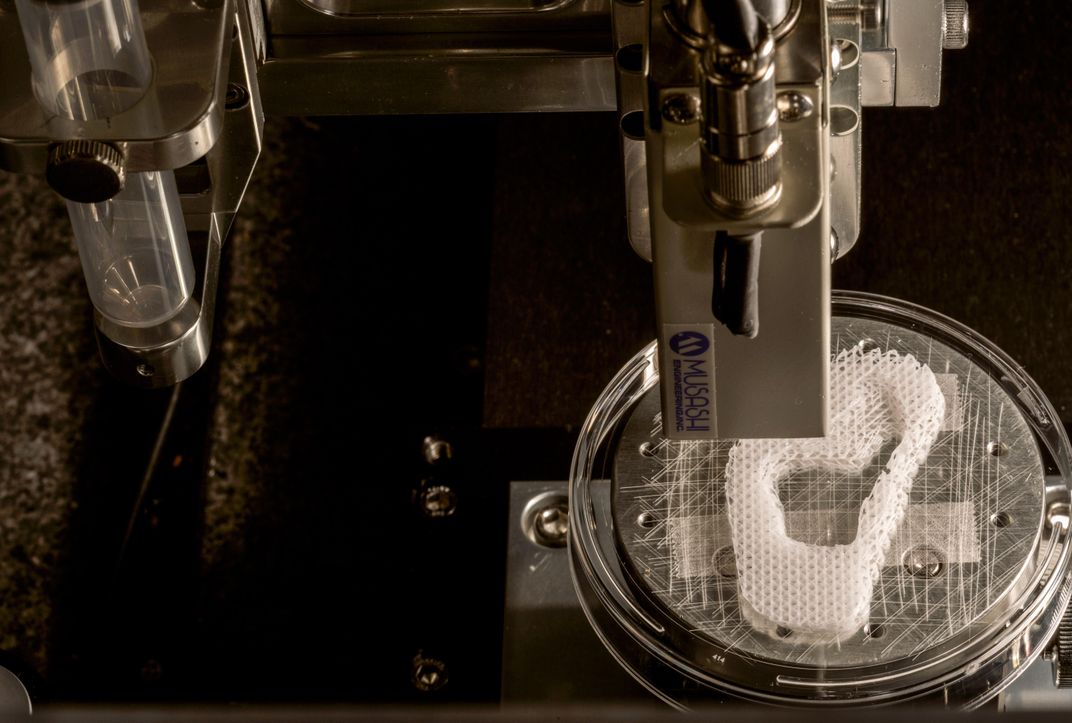
A researcher named Young-Joon Seol met me at the door to a room marked “Bioprinting.” Young-Joon, tousled-haired and wearing plastic-framed eyeglasses, grew up in South Korea and trained in mechanical engineering at a university in Pohang. At Wake Forest, he is part of a group that works with the lab’s custom-built bioprinters, powerful machines that operate in much the same way as standard 3-D printers: An object is scanned or designed using modeling software. That data is then sent to the printer, which uses syringes to lay down successive coats of matter until a three-dimensional object emerges. Traditional 3-D printers tend to work in plastics or wax. “What’s different here,” Young-Joon said, nudging his eyeglasses up his nose, “is that we have the capability to print something that’s alive.”
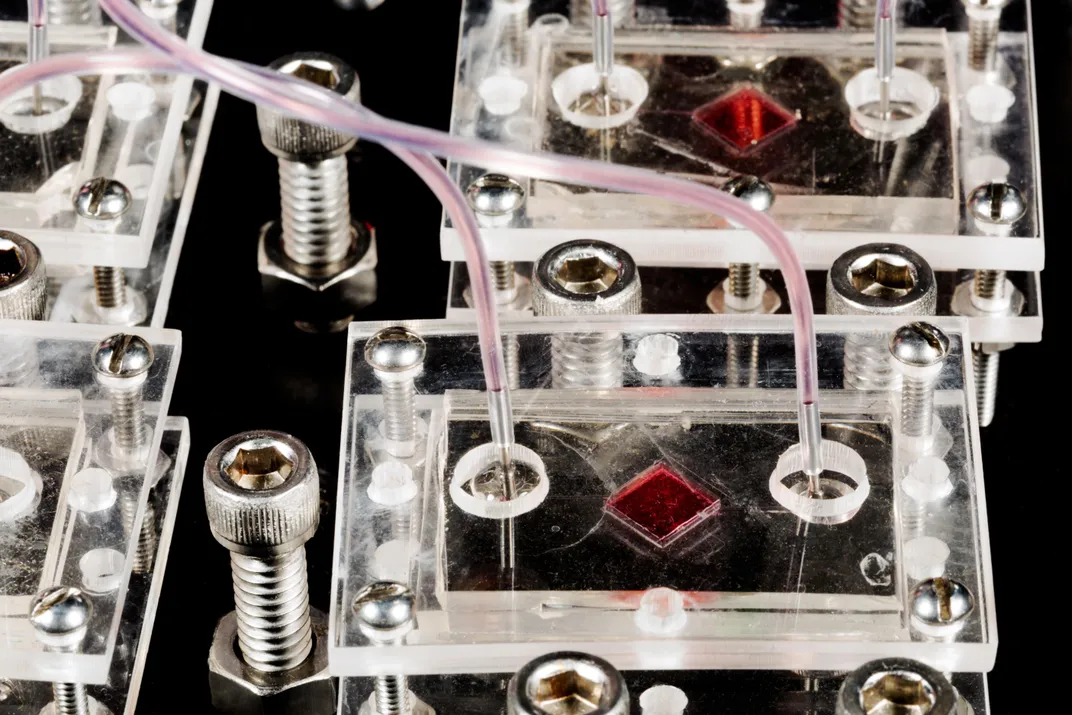
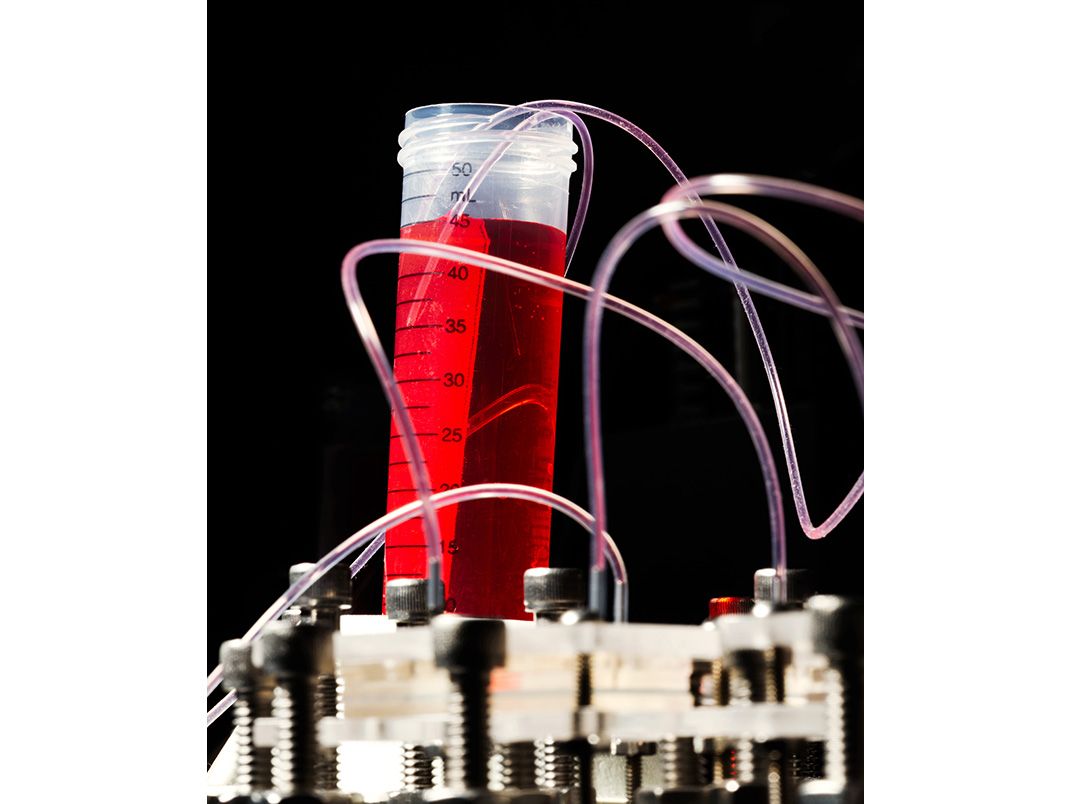
He gestured at the machine to his right. It bore a passing resemblance to one of those claw games you find at highway rest stops. The frame was heavy metal, the walls transparent. Inside were six syringes arranged in a row. One held a biocompatible plastic that, when printed, would form the interlocking structure of a scaffold—the skeleton, essentially—of a printed human organ or body part. The others could be filled with a gel containing human cells or proteins to promote their growth.
As the scaffold is being printed, cells from an intended patient are printed onto, and into, the scaffold; the structure is placed in an incubator; the cells multiply; and in principle the object is implanted onto, or into, the patient. In time, the object becomes as much a part of the patient’s body as the organs he was born with. “That’s the hope, anyway,” Young-Joon said.
Young-Joon had programmed one of the printers to begin the process of creating the scaffold for a human ear, and the room filled with a comforting electronic thrum broken only by the occasional gasp from the printer—the release of the compressed air that kept it working. Peering through the glass case, I could see the scaffold coming into being by degrees—small, delicate, extremely earlike. Because the process would take hours to complete, Young-Joon handed me a finished version to handle. It was light; it rested on my palm like a butterfly.
The external structure of the ear is one of the first structures that the institute at Wake Forest (and other research centers) have tried to master, as a stepping stone toward more complicated ones. Wake Forest staffers have implanted bioprinted skin, ears, bone, and muscle on laboratory animals, where they grew successfully into the surrounding tissue.
To evangelists of bioprinting, who are increasing—the number of 3-D printers shipped to medical facilities is expected to double in the next five years—the trials are a harbinger of a world that is only now coming into focus: a world where patients order up replacement parts for their body the same way they used to order a replacement carburetor for their Chevy.
“Think about it like the Dell model,” said Anthony Atala, a pediatric urologist and the institute’s director, referring to the computer company’s famous “direct” relationship model between consumer and manufacturer. We were sitting in Atala’s office on the fourth floor of the research center. “You’d have companies that exist to process cells, create constructs, tissue. Your surgeon might take a CT scan and a tissue sample and ship it to that company,” he said. A week or so later, an organ would arrive in a sterile container via FedEx, ready for implantation. Presto, change-o: A new piece of me—of you—made to order.
“What’s interesting is that there are no real surgical challenges,” Atala said. “There are only the technological hurdles that you’ve got to overcome to make sure the engineered tissue functions correctly in the first place.”
We’re getting close, with “simple” organs like skin, the external ear, the tube-like trachea. At the same time, Atala can’t help but look to what might come next. At his most sanguine, he likes to envision a vast bioprinting industry capable of cranking out big and complex organs without which the body would fail, like the liver or the kidney. An industry that could make traditional transplants—with their long, often fatal wait times and the ever-present risk of organ rejection—completely obsolete.
It would be a full-on medical revolution. It would change everything. And if he’s right, Wake Forest, with its purring bioprinters and fleshy ears and multicolored veins and arteries could be where it all starts.
The idea that a broken piece of ourselves might be replaced with a healthy piece, or a piece from somebody else, stretches back centuries. Cosmas and Damian, patron saints of surgeons, were alleged to have attached the leg of a recently deceased Ethiopian Moor onto a white Roman in the third century A.D., a subject depicted by numerous Renaissance artists. By the 20th century, medicine had at last begun to catch up with the imagination. In 1905 the ophthalmologist Eduard Zirm successfully cut a cornea from an injured 11-year-old boy and emigrated it into the body of a 45-year-old Czech farm laborer whose eyes had been damaged while he was slaking lime. A decade later, Sir Harold Gillies, sometimes called a founding father of plastic surgery, performed skin grafts on British soldiers during World War I.
But the first successful transplant of a major organ—an organ vital to human function—didn’t happen until 1954, when Ronald Herrick, a 23-year-old from Massachusetts, donated one of his healthy kidneys to his twin brother, Richard, who was suffering from chronic nephritis. Because the identical Herrick twins shared the same DNA, Joseph Murray, a surgeon at Peter Bent Brigham Hospital (today known as Brigham and Women’s), was convinced he’d found an end-run around the problem of organ rejection.
In his autobiography, Surgery of the Soul, Murray recalled the moment of triumph. “There was a collective hush in the operating room as we gently removed the clamps from the vessels newly attached to the donor kidney. As blood flow was restored, Richard’s new kidney began to become engorged and turn pink,” he wrote. “There were grins all around.” With the Herricks, Murray had proved an essential point about our biological myopia, an insight that drives so much of today’s cutting-edge bioengineering: There is no substitute for using a patient’s own genetic material.
As surgical science improved along with the immunosuppressive treatments that allowed patients to accept foreign organs, what once seemed all but out-of-reach became reality. The first successful pancreas transplant was performed in 1966, the first heart and liver transplants in 1967. By 1984, Congress had passed the National Organ Transplant Act, which created a national registry for organ matching and sought to ensure that donor organs were being fairly distributed. In hospitals across the country, doctors broke the news as gently as they could—The supply simply is not meeting the demand, you’ll have to hang on—and in many cases they watched as patients died waiting for their names to tick to the top of the list. This basic problem has not gone away. According to the U.S. Department of Health & Human Services, 21 people die each day in this country alone waiting for an organ. “For me, the demand wasn’t an abstract thing,” Atala told me recently. “It was very real, it was heartbreaking, and it drove me. It drove all of us to find new fixes.”
Atala, who is 57, is thin and slightly stoop-shouldered, with a shock of brown hair and an easy affability—he encourages everyone to call him Tony. Born in Peru and raised in Florida, Atala earned his M.D. and specialized training in urology at the University of Louisville. In 1990, he received a two-year fellowship with the Harvard Medical School. (Today, at Wake Forest, he still blocks off at least one day a week to see patients.) At Harvard he joined a new wave of young scientists who believed one solution to the organ donor shortage might be the creation, in a laboratory, of replacement parts.
Among their first big projects was to try to grow a human bladder—a relatively big organ, but a hollow one, fairly simple in its function. He used a suturing needle to stitch together a biodegradable scaffold by hand. Later, he took urothelial cells from the bladder and urinary tract of a potential patient and multiplied them in the lab, then he applied the cells to the structure. “It was like baking a layer cake,” Atala told me. “We did it one layer at a time. And once we had all the cells seeded, we then put them back into an incubator, and we let it cook.” Within a few weeks, what emerged was a little white orb, not so dissimilar-looking from the real thing.
Between 1999 and 2001, after a series of tests on dogs, custom-grown bladders were transplanted into seven young patients suffering from spina bifida, a debilitating disorder that was causing their bladders to fail. In 2006, in a much-heralded paper in the Lancet, Atala announced that, seven years on, the bioengineered bladders were working remarkably well. It was the first time lab-grown organs had been successfully transplanted in humans. “This is one small step in our ability to go forward in replacing damaged tissues and organs,” Atala said in a press release at the time, echoing the words of Neil Armstrong. It was a representative example of one of Atala’s primary gifts. As David Scadden, the director of the Center for Regenerative Medicine at Massachusetts General Hospital and the co-director of the Harvard Stem Cell Institute, told me, Atala has “always been a visionary. He’s always been quite bold, and quite effective in his ability to draw attention to the science.”
Bladders were an important milestone, but they didn’t rank particularly high in terms of patient demand. Moreover, the multi-stage approval process required by the U.S. Food and Drug Administration for such procedures can take time. Today the bladders Atala engineered haven’t yet received approval for widespread use. “When you’re thinking about regenerative medicine, you’ve got to be thinking not just about what’s possible, but what is needed,” Atala told me. “You’ve got to think, ‘I only have this much time, so what’s going to make the greatest possible impact on the most lives?’”
For Atala, the answer was simple. About eight out of ten patients on a transplant list needs a kidney. According to a recent estimate, they wait an average of four and a half years for a donor, often in serious pain. If Atala really wanted to solve the organ shortage crisis, there was no way around it: He’d have to deal with the kidney.
From its origins in the early 1980s, when it was viewed largely as an industrial tool for building prototypes, 3-D printing has grown into a multibillion-dollar industry, with an ever-widening range of potential applications, from designer shoes to dental crowns to homemade plastic guns. (Today, you can walk into an electronics store and purchase a portable 3-D printer for less than $500.) The first medical researcher to make the leap to living matter was Thomas Boland who, while a professor of bioengineering at Clemson University, in South Carolina, in 2003 filed for a patent on a customized inkjet printer capable of printing human cells in a gel mixture. Soon, researchers like Atala were tinkering with their own versions of the machine.
For Atala, the promise of bioprinting had everything to do with scale. Though he’d successfully grown an organ in a lab and transplanted it into a human, the process was incredibly time-intensive, precision was lacking, reproducibility was low, and the possibility of human error omnipresent.
At Wake Forest, where Atala became the institute’s founding director in 2004, he began experimenting with printing skin, bone, muscle, cartilage and, not least, kidney structures. Within a few years he was confident enough in his progress to show it off. In 2011, Atala gave a TED Talk on the future of bioengineered organs that has since been viewed more than two million times. Wearing pleated khakis and a courtly striped button-down shirt, he spoke of the “major health crisis” presented by the organ shortage, partly a result of our longer lifespans. He described the medical challenges that innovation and dogged lab work had summarily conquered: devising the best biomaterials for use in scaffolds, learning how to grow organ-specific cells outside the human body and keep them alive. (Some cells, he explained, like those of the pancreas and the liver, remained stubbornly difficult to grow.)
And he spoke about bioprinting, showing a video of a few of his printers at work in the lab and then revealing a printer behind him on the stage, busy building a pinkish spherical object. Toward the end of his talk, one of his colleagues emerged with a large beaker filled with a pink liquid.
While the crowd sat in silence, Atala reached into the beaker and pulled out what appeared to be a slimy, oversized bean. In a masterly display of showmanship, he held the object forward in his cupped hands. “You can actually see the kidney as it was printed earlier today,” he said. The crowd broke into spontaneous applause. The next day, the wire news organization Agence France-Presse gushed in a widely disseminated article that Atala had printed a “real kidney” on a machine that “eliminates the need for donors when it comes to organ transplants.”
The future was coming.
And then it wasn’t.
In fact, what Atala had held on stage wasn’t a working human kidney. It was inert, an extremely detailed model, a taste of what he hoped and thought bioprinting would one day bring. If you watched the presentation carefully, you could see that Atala never promised that what he held was a working organ. Still, critics pounced on what they viewed as a high-grade exercise in special effects.
Last year, Jennifer Lewis, a materials scientist at Harvard and a leading researcher in bioprinting (her specialty is engineering vascularized tissues) seemed to criticize Atala in an interview with the New Yorker. “I thought it was misleading,” she said, referring to the TED Talk. “We don’t want to give people false expectations, and it gives the field a bad name.”
In the aftermath of the TED Talk, Wake Forest issued a press release stressing that it would be a long time before a bioprinted kidney could come to market. When I asked Atala whether he’d learned anything from the controversy, he declined to comment on it directly, pointing instead to why he dislikes putting a time stamp on any particular project. “We do not want to give patients false hope,” he told me.
The dust-up was neatly illustrative of one of the central challenges faced by researchers throughout the field of regenerative medicine: You want to stoke enthusiasm about what’s possible, because enthusiasm can translate to press, funding and resources. You want to inspire the people around you and the next generation of scientists. But you don’t want to misrepresent what’s realistically within reach.
And when it comes to big, complicated organs, the field still has a way to go. Sit down with a pencil and a piece of paper and you could hardly dream up something more architecturally or functionally complex than the human kidney. The interior of the fist-size organ is made up of solid tissues traversed by an intricate highway system of blood vessels, which measure as little as 0.010 millimeters in diameter, and approximately a million tiny filters known as nephrons, which send healthful fluids back into the bloodstream and waste down to the bladder in the form of urine. To bioprint a kidney, you’d have to be able to cultivate and introduce not only functioning kidney cells and nephrons, you’d also need to have mastered how to populate the organ with a vasculature to keep the organ fed with the blood and nutrients it needs. And you’d have to build it all from the inside out.
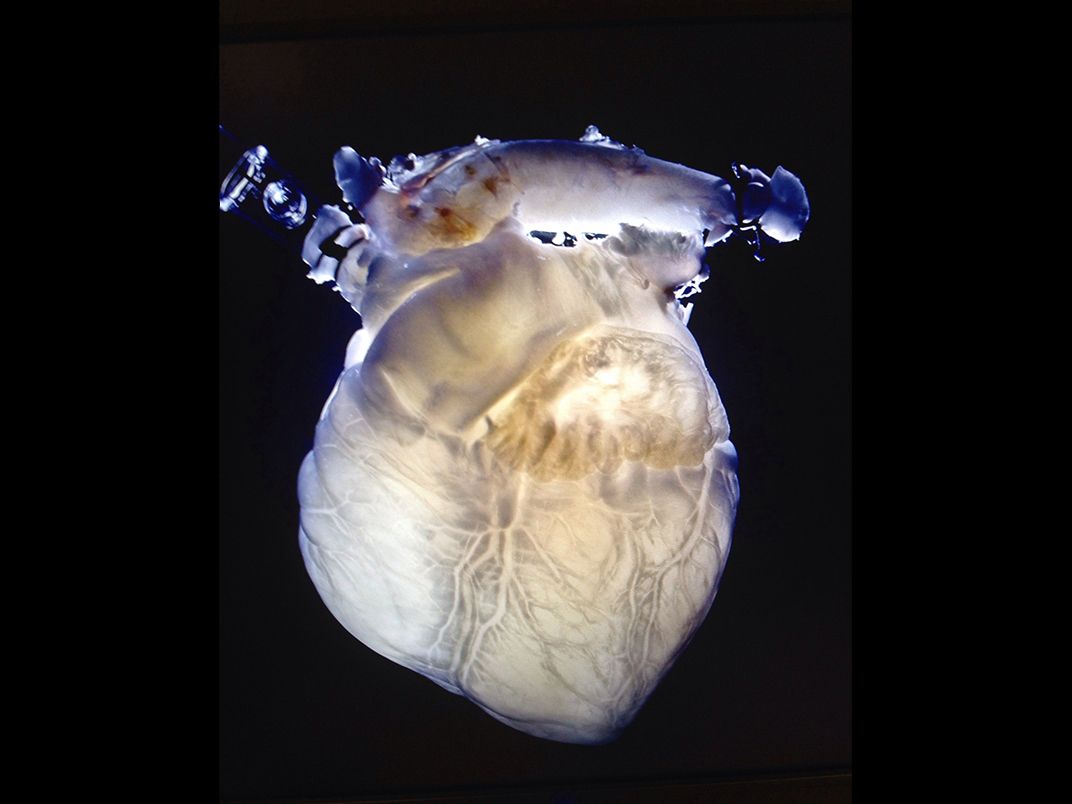
Which is why many researchers are exploring options that don’t include printing those structures from scratch but instead try to use those already designed by nature. At the Texas Heart Institute, in Houston, Doris Taylor, the director of the institute’s regenerative medicine research program, is experimenting with decellularized pig hearts—organs that have been stripped of muscle and all other living tissue cells in a chemical bath, leaving only the underlying collagen matrix. A decellularized organ is pale and ghostly—it resembles a glow stick drained of the solution that once made it glow. But crucially, the process leaves the interior architecture of the organ intact, vasculature and all.
Taylor hopes one day to use decellularized pig hearts, repopulated with human cells, for transplant in human patients. So far, her team has injected the hearts with live bovine cells and inserted them into cows, where they successfully beat and pumped blood alongside the cows’ original, healthy heart. For Taylor, this approach skirts the challenges of finding ways to print at the incredibly fine resolution that vascular networks require. “The tech is going to have to improve a great deal before we’re able to bioprint a kidney or a heart, and get blood to it, and keep it alive,” Taylor says.
Researchers at Wake Forest are also experimenting with decellularized organs from both animal and human cadavers. Indeed, although Atala sees the replacement kidney as his Holy Grail, he doesn’t pretend that building one will be anything but an incremental process, undertaken from a variety of angles. So while researchers at the institute and elsewhere work to refine printing the organ’s external structure and internal architecture, they’re also experimenting with different ways to print and grow blood vessels. At the same time, they’re honing techniques to cultivate the living kidney cells necessary to make it all work, including a new project to propagate kidney cells taken from a biopsy of a patient’s healthy tissue.
When we talked, Atala emphasized that his goal is to get a functioning, engineered large organ into a human being who desperately needs it, whether that organ was bioprinted or not. “Whatever technology it takes to get there,” he said.
And yet he was quick to point out that the way you get there isn’t unimportant: Ultimately, you want to lay the foundation for an industry that will ensure that no one—whether in the coming decades or in the 22nd century, depending on your level of optimism—will ever want for a life-saving organ again. To do that, you can’t go at it by hand.
“You’ll need a device that’s able to create the same type of organ time and time again,” Atala told me. “Just like it was machine-made.”
One afternoon, I stopped by the desk of John Jackson, an associate professor at the institute. Jackson, 63, is an experimental hematologist by trade. He came to Wake Forest four years ago, and likened the move to the institute, with all its next-generation tech, as “going back to school all over again.”
Jackson oversees the development of a skin-cell printer, which is designed to print a range of living skin cells directly onto a patient. “Say you have an injury to your skin,” Jackson suggested. “You’d scan that wound to get the exact size and shape of the defect, and you’d get a 3-D image of the defect. You could then print the cells”—which are grown in a hydrogel—“in the exact shape you need to fit the wound.” Right now, the printer can lay down tissues at the top two layers of skin, deep enough to treat—and to heal—most burn wounds. Down the line, the lab hopes to print deeper beneath the skin’s surface and to print more complicated layers of skin, including adipose tissue and deep-rooted hair follicles.
Jackson estimated clinical trials could start in the next five years, pending FDA approval. In the meantime, his team had been busy testing the skin printer on pigs. He unscrolled a large poster, which was divided into panels. In the first was a detailed photograph of a square wound, about four inches on one side, that technicians had cut on a pig’s back. (The pigs had been put under general anesthesia.) That same day, the researchers had printed cells directly onto the wound, a process that took about 30 minutes. In the post-printing photographs, you could make out a discrepancy in color and texture: The area was grayer and duller than natural pig flesh. But there was little puckering, no raised or ridged scar tissue, and, in time, the gel more or less completely melded into the surrounding skin.
The skin-cell printer is one of several active projects at the institute that receives funding from the U.S. Department of Defense, including tissue regeneration initiatives for facial and genital injuries, both of which have been endemic among American soldiers injured in recent wars. Last year, researchers led by Atala announced the successful implantation of vaginas engineered using the patients’ own cells in four teenagers suffering from a rare reproductive disorder called Mayer-Rokitansky-Küster-Hauser syndrome. Wake Forest is also testing lab-grown and decellularized cadaver penises and anal sphincters on animals, with the hope of starting human trials in the next five years.
The Peripheral, the new novel by the futurist William Gibson, who coined the term “cyberspace” and foresaw most of the digital revolution, takes place at a time when humans are able to “fab”—essentially 3-D print—anything they need: drugs, computers, clothing. They are constrained only by their imagination. And yet hunched over Jackson’s poster, I found myself thinking that even Gibson hadn’t predicted this: living flesh, on demand.
I walked over to Atala’s office. Sunlight splashed across the floor and a tall set of bookshelves, which displayed photos of Atala’s two young sons and several copies of his textbook, Principles of Regenerative Medicine.
He’d been in the operating room all morning (he’s also the medical school’s chairman of urology) and did not expect to head back home until late in the evening, but he was cheery and burbling over with energy. I asked him if he ever considered giving up his practice and focusing solely on research.
He shook his head. “At the end of the day, I went into medicine to take care of patients,” he said. “I love having that relationship with families and patients. But equally important, it keeps me in touch with what the need is. Because if I see that need firsthand, if I can put faces to the problem—well, I know I’ll keep working on it, keep trying to figure out.”

The Ageless Generation