Earth May Have Become Magnetic After Eating a Mercury-Like Object
Swallowing a sulfur-rich protoplanet could help explain two lingering mysteries in the story of Earth’s formation
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/5a/30/5a30b786-9e99-4b33-ab5a-bec39a9a9326/mdis_global_enhancedcolor_map_rot_140globebright.jpg)
In its infancy, Earth may have swallowed a planet similar to Mercury, but much bigger. This early meal could explain the puzzling makeup of Earth’s layers, and it could account for the magnetic field that makes life here possible.
“We think we can hit these two birds with one stone,” says Bernard Wood, a geochemist at the University of Oxford who reported the idea this week in the journal Nature.
If it seems incredible that in 2015 we still don’t know how our world formed, consider how difficult it is to peek at its interior. The longest, hardiest drills yet made cannot bore beyond Earth’s thin outer crust. Natural channels of hot rock helpfully bring up materials to the surface from the deeper mantle layer for us to study, but even these columns, hundreds of miles long, seem shallow when we think of the planet’s center more than 3,700 miles below us. Piecing together Earth’s history is therefore a bit like trying to guess how a cake was baked by tasting the icing and perhaps a few stray crumbs. There’s still plenty of room for new evidence and new ideas.
“It’s exciting time to be in the field,” says geochemist Richard Carlson of the Carnegie Institution of Washington. “A lot of things are coming out of studies of the deep Earth that we don’t understand very well.”
The traditional view of how Earth came together starts with space debris clumping. Rocks resembling the stony meteors that still rain down on us today glommed together into ever-bigger chunks. Squeezed, pummeled and heated, a growing rubble heap ultimately melted and then cooled, forming layers slowly over billions of years. Geological crumbs studied in the 1980s helped to corroborate this story. With the exception of certain metals such as iron, most of which is thought to have sunk to Earth's core, terrestrial rocks seemed to be made of pretty much the same stuff as chondrites, a particular group of stony meteors.
Then about a decade ago, Carlson found room for doubt, after comparing Earth rocks and space rocks using better instruments. His team investigated two rare elements with unusual names and magnetic personalities: neodymium, an ingredient in the magnets used in hybrid cars and large wind turbines, and samarium, common in headphone magnets. Terrestrial samples contained less neodymium relative to samarium than chondrites, the researchers found.
This small discrepancy of only a few percent was still difficult to explain. Perhaps, Carlson speculated, a cooling Earth formed layers much faster than previously thought, in tens of millions of years instead of billions. An upper layer that formed quickly would be depleted in neodymium, balanced by a lower layer that hid the missing element deep in the mantle. However, no evidence has been found of this secret reservoir. Its tendency to remain stubbornly stuck at depth is difficult to explain, given that the mantle churns like boiling soup, often bringing its ingredients to the surface as it creates volcanoes. And if the moon was born when a planetary body smashed into Earth, as is commonly thought, the melting caused by that impact should have mixed the reservoir back into the mantle.
Instead of trying to account for hidden neodymium, a second group of scientists came up with a way to get rid of it. They imagined a crust enriched in neodymium growing on the chondritic rocks out of which Earth was made. Collisions between these objects could have scraped away much of this outer layer, making neodymium rarer.
But there are problems with this view, too. No meteorites have ever been found with compositions similar to the eroded debris. Also, that sloughed-off skin would have taken with it much of the Earth’s heat. Uranium, thorium and other radioactive materials, which we know are responsible for our planet's heat, would have also ended up in the removed layer.
“About 40 percent of Earth's heat-producing elements would be lost to space,” says Ian Campbell, the geochemist at the Australian National University.
Hoping to hold on to these critical elements, Wood decided to tweak the chemistry of Earth in its youth. He took inspiration from one of the stranger planets in our solar system: Mercury. Chemically speaking, the closest planet to the sun is a hellish place loaded with actual brimstone, known to modern science as sulfur. How would layers form in a young Earth if the planet looked more like Mercury? To answer this question, Wood added sulfur to mixtures of elements meant to simulate the composition of primitive Earth. He cooked the mock planets at temperatures as hot as burning jet fuel and pounded them with a piston to pressures about 15,000 times that inside a typical household pressure cooker.
Dosed with enough sulfur, the miniature proto-worlds buried neodymium as they formed layers—not in their fake mantles, but deeper still in their fake cores. Neodymium trapped in the core for good could account for Carlson’s anomaly. This extra sulfur could have come from a Mercury-like object that struck the growing Earth early on, perhaps even the same object thought to have formed the moon, suggests Wood.
“We would need a body 20 to 40 percent the size of Earth.” It's also possible that Earth grew at the start from a kernel made not from chondrites but from other space rubble rich in sulfur. Either way, this cosmic storyline could have set the stage for the rise of life on Earth. That’s because sulfur also would have helped to draw uranium and thorium into the core. The added heat from these radioactive elements could help to churn the outer part of the core, and this vigorous motion of molten metal is thought to give rise to the currents that in turn generate Earth’s magnetic field.
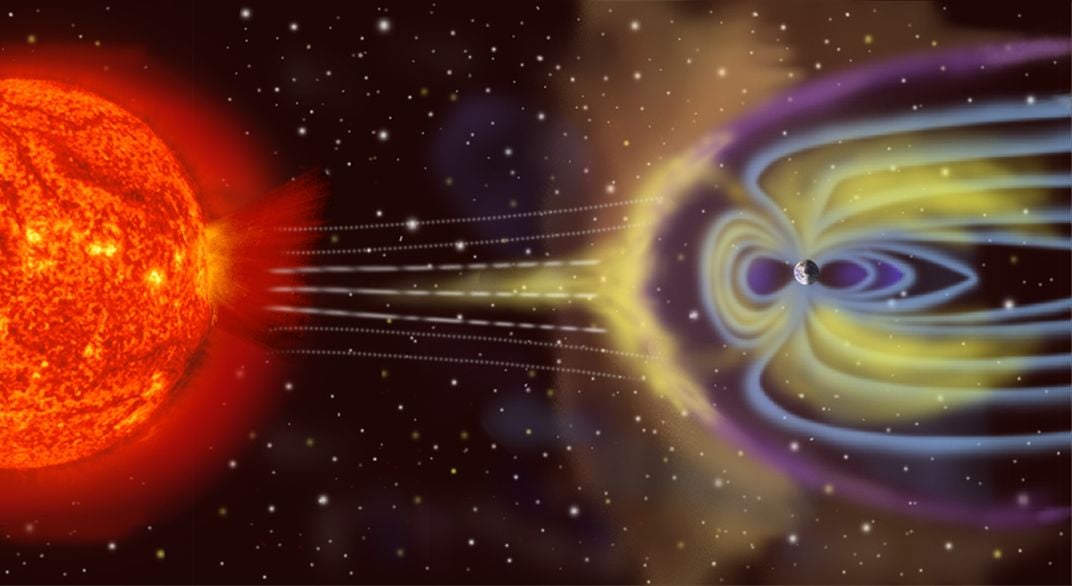
Without magnetism, sea turtles and sea captains wouldn’t be able to navigate—or even exist. Life wouldn’t have been possible on the planet's surface without the protection the field provides against high-energy particles streaming out of the sun.
Wood’s colleagues describe his theory as plausible. But like the other origin stories that have been written in recent years about Earth, it is far from definitive. For one thing, the temperatures and pressures reached in the experiment, as extreme as they were, fell far short of the conditions inside proto-Earth. For another, studies of how earthquakes travel through the planet’s interior have placed limits on how light the core can be, and dumping lots of sulfur in the center of the planet could put the core uncomfortably close to those limits.
To strengthen his case, Wood plans to scour the periodic table for other elements with mysterious abundances that could be explained by adding sulfur to the primordial mix. Given the history of the field, it’s going to take a lot to convince skeptics like Bill McDonough, a geochemist at the University of Maryland. “I put this idea at well below the 50 percent chance of being right,” he says.