Harmful Bacteria Masquerade as Red Blood Cells to Evade the Immune System
Studying the stealthy strategy could help researchers develop new treatments for group A strep infections, which kill more than 500,000 people each year
:focal(1832x1227:1833x1228)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/49/e0/49e03f85-6664-4980-a5f1-1974838f3717/gettyimages-179796759.jpg)
Even single cells must sometimes be masters of disguise.
Various types of harmful bacteria, for example, masquerade as human cells to evade the immune system, blanketing their surfaces with molecules that resemble our own. The clever trick effectively gives the pathogens “cloaks of invisibility,” says David Gonzalez, a biochemist and microbiologist at the University of California, San Diego.
Now, Gonzalez and his team have discovered a new form of this microbial mimicry that’s especially macabre. To avoid being snuffed out by the immune system, the bacteria that cause strep throat tear apart red blood cells and then dress themselves in the debris, as reported today in the journal Cell Reports.
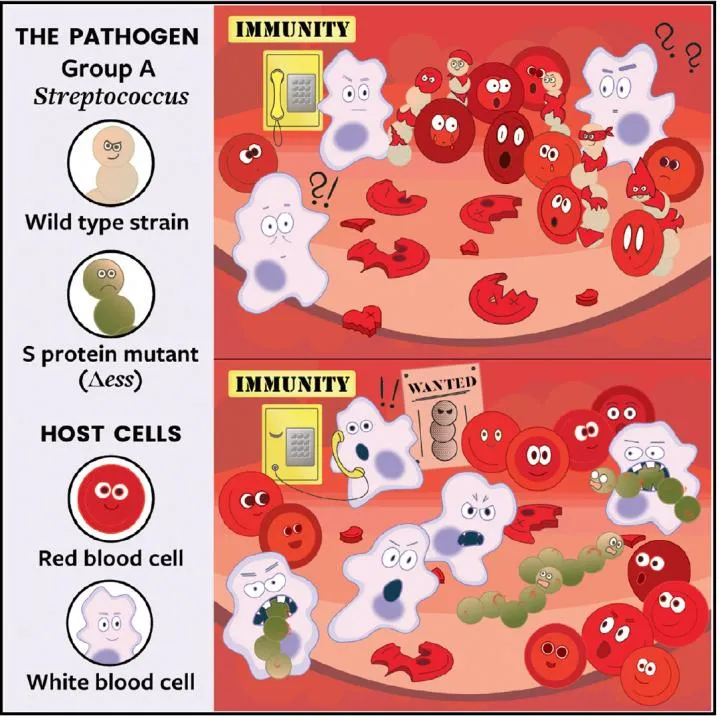
When this strategy works, the bacteria, called Group A Streptococcus (group A strep), remain concealed while they wreak havoc on the body, the study’s mouse experiments show. But when a protein in the bacteria responsible for the sanguine disguise is snipped out of the strep genome, the microbes are left exposed, allowing the immune system to attack the pathogens and prevent a potentially deadly infection.
Understanding the biology behind group A strep’s bloody disappearing act might aid the search for new drugs that “uncloak the bacteria so they can be effectively cleared or killed,” says Martina Sanderson-Smith, a molecular microbiologist at the University of Wollongong in Australia who wasn’t involved in the study. “This is an example of discovery science at its best.”
Among pathogens, group A strep is something of a Swiss Army knife. These versatile microbes can colonize the skin, throat, genitals and more, and they infect hundreds of millions of people each year. Many infections don’t progress further than an annoying rash or sore throat, but under more dire circumstances, the bacteria can threaten lives with conditions like rheumatic fever, toxic shock syndrome or flesh-eating disease.
Though antibiotics against group A strep exist, resistance to some drugs is growing among strains worldwide, and no vaccines are commercially available. Finding new treatments to combat these pathogens, Gonzalez says, could prevent some of the 500,000-plus deaths they cause annually.
Much of how group A strep manages to outsmart the body’s defenses remains mysterious. To better understand the bacteria’s elusive ways, Gonzalez and his lab have spent the past few years studying the suite of molecules produced by the pathogen during infection. Some of these molecules stick to red blood cells, including a handful of proteins that can rip the cells to shreds.
But when the researchers used nanoparticles coated with pieces of blood cells as bait, they snared a new protein called S protein. Instead of tearing blood cells apart, this molecule allowed the bacteria to cling to the pieces left behind.
At first, the seemingly innocuous stickiness of S protein baffled Gonzalez and his team. But they soon realized it might allow the bacteria to pass as the very cells they’d destroyed—the microscopic equivalent of wolves in sheep’s clothing.
The deception is an unusual tactic, but an effective one, says co-first author Anaamika Campeau, a biochemist in Gonzalez’s lab. To hide any features that might incriminate group A strep as foreign invaders, the microbes plaster themselves with pieces of cells the immune system sees all the time and knows not to attack, she explains. “Once we kind of came to that idea, it all sort of fell into place.”
The interaction between group A strep and red blood cells was so strong that the bacteria turned bright crimson when plopped into solutions of human blood. Immune cells, flummoxed by the bloody disguise, largely failed to capture and kill the would-be invaders.
When the researchers generated a mutant strain of the bacteria that couldn’t make S protein, however, it struggled to disguise itself, turning only faintly pink in the presence of blood. The modified pathogens didn’t fool the immune cells, which quickly gobbled up their targets.
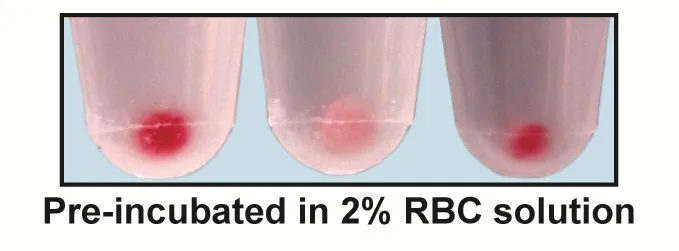
To test the potency of S protein’s evasive effects, the researchers then injected each of the two bacterial strains into mice. While nearly all the animals infected with typical group A strep rapidly lost weight and died, every mouse that got the mutant microbes survived and remained at a healthy size.
The difference was so striking that, at first, Gonzalez and his team were certain they’d made a mistake. But even with more than the lethal dose of mutant bacteria, he says, “the mice were still just as happy as can be.”
Microbes mimicking host cells isn’t a new biological trick, says Tiara Pérez Morales, a molecular microbiologist at Benedictine University who wasn’t involved in the study. But the new study puts a plot twist on an old story. “They’re putting on a costume and pretending they’re red blood cells,” she says. “I don’t think I can think of anything else like it.”
The loss of S protein so severely hamstrings the bacteria that the molecule could be an appealing target for new drugs in the future, Sanderson-Smith says. Blocking the protein’s activity during infection would essentially leave the bacteria in the buff, helping immune cells identify and destroy the pathogens.
Gonzalez hopes that S-protein-based treatments will go beyond simply unmasking group A strep. After receiving a hefty dose of the mutant bacteria, mice began to churn out immune proteins—an indication, he says, that the altered strain had alerted the body to its presence without causing it serious harm. The microbes, it seemed, had become a living vaccine.
The team then conducted a final experiment, dosing mice with either the mutant bacteria or a saline solution before reinfecting them with normal group A strep three weeks later. While 90 percent of the animals given saline died within ten days, seven out of the eight mice that had first been exposed to the mutant strain pulled through.
“That was exciting to see,” says Pérez Morales, adding that the findings could prove especially significant if they can be repeated in other members of the Streptococcus genus, which includes several other pathogens that appear to also make S protein.
But Pérez Morales and Sanderson-Smith caution that a lot more needs to happen before human vaccination can be considered. Microbes and the immune cells they parry with are extremely complex and ever-evolving, and what works in mice doesn’t always translate into people. Other vaccine candidates have shown promise over the years, but they’ve encountered several hurdles that have kept them out of the clinic.
Still, as the issue of antibiotic resistance continues to balloon worldwide, this study highlights the importance of taking creative new approaches to treatment. “We need alternatives,” Pérez Morales says. “We can’t just keep hitting this problem with antibiotics.”
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/10172852_10152012979290896_320129237_n.jpg)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/10172852_10152012979290896_320129237_n.jpg)