How to Grow a Nanogarden
In a lab at Harvard University, Wim Noorduin cultivates microscopic crystalline flowers in glass beakers
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/2e/c6/2ec6fada-14b8-4ef5-8c99-9ef3ff28d2f5/blue-nanoflowers-wim-noorduin.jpg)
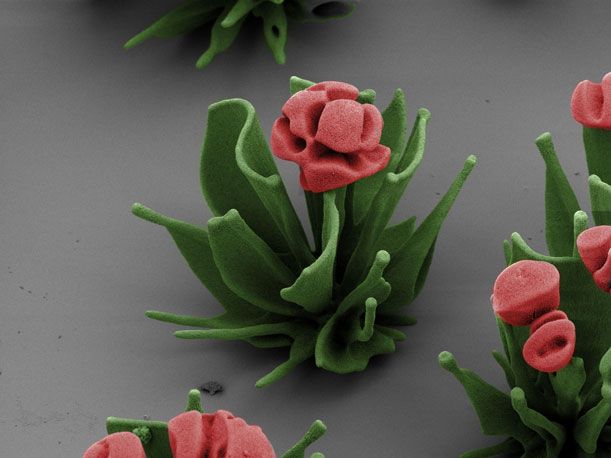
Wim Noorduin has a green thumb—but, he doesn’t grow your standard garden-variety roses, tulips and other flowers. The postdoctoral fellow at Harvard University’s School of Engineering and Applied Sciences, instead, tends to microscopic “buds” that he carefully cultivates in his lab. The blooms—delicate and fragile—are made out of crystal.

“The technique is remarkably easy: fill a beaker with a solution that has a salt and a silicon compound dissolved in it. Put in a glass slide or a bit of metal to act as the soil on which the crystal ‘plants’ will grow. Allow carbon dioxide from the air to diffuse into the solution, triggering a simple reaction that causes the dissolved chemicals to come out of the solution and form a solid crystal—one that is curvy, rather than jagged,” the Boston Globe explained in a recent article. Add a little dye here and there and what results are crystal growths that resemble the leaves and petals of flowers.
The Globe‘s peek into Noorduin’s project was prompted by the journal Science and its decision to feature the scientist’s “nanoflowers” in its pages. Science published a paper authored by Noorduin and three of his colleagues describing the creative endeavor and an essay about the work.

Previously, scientists have grown structures that resemble flora from materials like zinc oxide before, but what is unique about Noorduin is his ability to manipulate the growth of barium carbonate and silicate to his liking. He and his team understand what conditions produce what shapes, so much so that they are able “to design the resulting shapes at will and to combine different growth conditions to generate even more complex shapes,” writes Elias Vlieg, a chemistry professor at Radboud University in the Netherlands, in Science. “Rather than selecting one set of conditions and letting the system evolve passively, the authors change the process conditions actively, allowing the construction of elements such as stems, vases, branches, and leaves.”
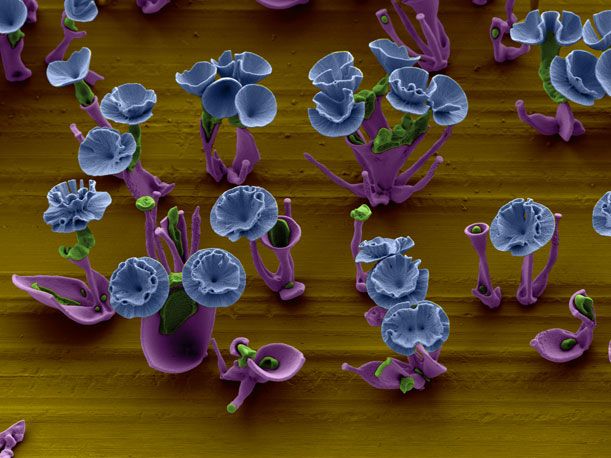
For example, to produce a vase, Noorduin fluctuates the amount of carbon dioxide that enters his solution, simply by covering or uncovering the beaker. The supply of gas controls the thickness of the vase. Within the vase, he then places a stem; while cultivating it, he says he adds a “pulse of CO2″ so that the stem opens into a bud. If he wants to build a rose, the scientist-cum-artist adjusts the pH level of the solution. This way, the petals curl up and form spirals, he explains in an email. In an electron micrograph, Noorduin’s garden is naturally black and white in color, but he adds artificial hues to the images to distinguish the plants’ leaves and stems from their blossoms.

To really drive home the miniscule scale of his creations, Noorduin planted a field of flowers on the steps of the Lincoln Memorial—on a penny.
Thus far, the scientist has experimented with floral patterns. He is curious, though, about other tiny architectures he might be able to construct. “Nature has many examples of remarkably diverse and complex mineralized architectures such as coral reefs at the macro scale, and the amazingly intricate skeletons of microorganisms such as acantharea at the micro scale,” he says. “Our aim is not so much to reproduce any particular shapes seen in nature. Rather, we’re inspired by a more fundamental observation: the diversity, hierarchy, and complexity of the patterns seems to be virtually unlimited.”

Noorduin’s repertoire will no doubt expand as he explores these limitless shapes. “More control will undoubtedly lead to structures that may be less artistically pleasing, but more technologically useful,” writes Vlieg.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/megan.png)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/megan.png)