Quick and Cheap DNA Sequencing On the Horizon?
A new technique reads DNA base by base by threading it through a tiny pore
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/minion-hero.jpg)
When the human genome was first sequenced about a decade ago, the achievement took years and cost $1 billion. Now, scientists and entrepreneurs are predicting that the task will soon take just under 6 hours, with a price tag of just $900. A company called Oxford Nanopore Technologies claims it will accomplish this feat using a device that can plug into your computer’s USB port.
The key to this remarkable rate of progress? A technology called nanopore sequencing, which allows researchers to determine the sequence of base pairs in an individual’s DNA without taking it apart.
Traditional DNA sequencing techniques involves making many copies of an individual’s genome, cutting it into millions of small fragments, and using radioactively-labelled bases to determine the exact sequence of the four bases that make up DNA—adenine, guanine, cytosine and thymine, often abbreviated A, G, C and T. Currently, sequencing using advanced versions of this technique takes about a week and costs roughly $18,000. The equipment takes up a lab bench and requires technicians to process the DNA sample before and after sequencing.
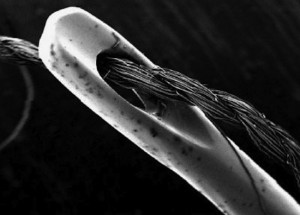
Threading DNA through a tiny hole called a nanopore, like a thread through a needle (above), may make DNA sequencing dramatically quicker and less expensive
As reported by Science, nanopore sequencing could speed up and simplify the process dramatically. The approach threads DNA continuously through microscopic protein pores—each so small that 25,000 can fit within the diameter of a human hair—and monitors the electrical current, which fluctuates slightly with each different type of base. As a result, the precise order of every single one of your roughly 3.2 billion bases might soon be able to be determined over the course of a mere business day.
Researchers have been working on developing the technique since the mid-1990s, but many technical challenges held up application of the approach. David Deamer, a biophysicist at the University of California, Santa Cruz and Daniel Branton, a cell biologist at Harvard, and other scientists worked out how to uncoil the DNA so that it can be moved single-file, found a pore large enough for the DNA to slide through and figured out how to use a particular enzyme to slow down the movement of the DNA so it can be read accurately.
The technique is not yet a finished product. The current error rate in sequencing using the technology is about 4 percent; some bases are read twice and others make it through the pore without being accurately detected. Oxford Nanopore declares that its technology, including the handheld MinION device, will hit the market shortly, but many are skeptical. Other groups have made claims before that inexpensive DNA sequencing was just over the horizon, but we still have yet to see it become a reality.
There is also the question of just how useful individualized DNA sequencing would be in medical applications even if it becomes available. As recently pointed out in the Wall Street Journal, gene therapy—medical treatment based on an individual’s own genetic data—has not lived up to expectations. The relationship between genes and health is far more complicated that first assumed.
Nevertheless, it’s clear that there are countless valuable applications for DNA sequencing. It has been heavily used in a range of fields, from biology to archaeology to criminal forensics. It’s even now becoming popular for the general public: DNA kits for testing paternity and revealing ancestry are available at Walmart, of all places.
But in predicting the time when complete DNA sequencing becomes economical on a wide scale, it’s not a matter of if, but when. Sequencing might abide by its own version of Moore’s Law, the famous rule for computing power, which dictates that processing speed doubles roughly every 18 months. We might not have the $900 DNA sequence as soon as private companies promise, but it’s hard to imagine not seeing it during our lifetimes.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/joseph-stromberg-240.jpg)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/joseph-stromberg-240.jpg)