By Studying Mouth Bacteria, Scientists Hope to Learn the Secrets of Microbiomes
Communities of bacteria and other microbes in the human mouth can help researchers learn how these groups of organisms affect human health
:focal(625x517:626x518)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/42/ce/42cec67b-ba1c-4cad-9fd1-013558a77a4d/g-habitats-of-the-mouth-alt.png)
If you’ve ever brushed your teeth or swished some mouthwash, they’ve been in your sight: the hundreds of billions of microorganisms—mostly bacteria—that live in the average human mouth. Dangling from the hard palate, burrowed in the nooks and crannies of the tongue and intertwined in the plaque on teeth are the many hundreds of species that make up the human oral microbiome.
For most, the bacteria in your mouth seem largely an inconvenience—critters all mixed together in a smelly goo, that must be flossed, brushed or rinsed away to keep your breath pleasant and gums healthily pink. But for Jessica Mark Welch of the Marine Biological Laboratory in Woods Hole, Massachusetts, and Gary Borisy and Floyd Dewhirst of the Forsyth Institute in Cambridge, Massachusetts, the oral microbiome is a wonder. Far from a jumbled mess of cells, it’s a varied, ordered ecosystem that can reveal larger truths about the ways microbes interact with one another—and how their interactions impact the environments they inhabit.
Charting the way microbes array themselves in the mouth could shed light on the ways communities of organisms organize themselves in a variety of ecosystems, the scientists say: from the pores of kitchen sponges to the surfaces within kelp forests. Understanding more about the microbial rules of engagement could help leverage microbiomes to improve health, or, more far afield, help solve technological challenges like making biofuel from switchgrass.
And of course, exploring the oral microbiome specifically can sharpen understanding of how some bacteria in the mouth keep us healthy—as key actors in normal metabolism—while others may be implicated in illnesses like gum disease, heart disease and cancer.
Mark Welch, Borisy and Dewhirst, who recently reviewed what’s known about the geographic distribution of species inhabiting the mouth in the Annual Review of Microbiology, have used genetic analysis and fluorescent imaging to map the microbes—from the chain-linked Streptococcus species that thrive on the tongue to the rod-shaped Corynebacteria that hang out in dental plaque to all the other bacteria that live among them.
Their work suggests that bacteria live in communities that are far more structured than previously believed. “I think we expected more big wads of bacteria,” says Mark Welch. “What was really a surprise was to see how organized they were. It tells us a lot about how they are working together.”
This interview has been edited for length and clarity.
Jessica, you are a geneticist. Gary, you’re a cell biologist. How did you end up studying bacteria in the mouth?
GB: We wanted to study microbiomes—communities of bacteria—the ways they organize themselves, and why that matters.
The mouth was not the first place we began. We started by looking in the natural environment, at microbes in a pond and in a marsh in Woods Hole. We also sampled the manmade environment: dollar bills, and the scum around the toilet bowl.
JMW: And what you find on the sponge in your kitchen sink! There are microbiomes everywhere, and they play an important role in ecosystems.
GB: But we realized rather early on that there was a big problem. When we collected our samples, we could see many individual organisms, but we weren’t really sure what we were looking at. The genomics database for most environments was sparse. No one had systematically sequenced the microbes we were seeing, so it was hard to identify them when we sampled them, much less understand the ways they worked together to make ecosystems.
And studying the mouth solved that problem?
GB: Yes. One reason for going to the mouth was the existence of this superb database that our coauthor Floyd Dewhirst and his colleagues at Forsyth had developed—the Human Oral Microbiome Database, which catalogs the genomes of hundreds of bacterial species found in the mouth. A lot of the organisms we would see if we started collecting bacteria from the mouth for our research were already identified and cultured, and the genomic information was being curated—all of this provided the foundation for the imaging work we wanted to do.
Also, from a craven perspective, it seemed it would be easier to get money to support this work if we did something related to humans.
JMW: Another thing that makes the mouth a fantastic environment to study is that the different microbial communities—the bacteria that grow on the different surfaces in the mouth—are so different from one another.
And yet they’re all in the same mouth, experiencing the same saliva, the same immune system, the same daily eating and sleeping schedule. You’re controlling for many of the factors that might influence the community. You can really compare the influence of the surfaces they’re living on, and their location in the mouth.
So what is this landscape of the mouth? Who lives where?
FD: The Human Microbiome Project defines nine sites in the mouth—the tongue, palate, tonsils, sub- and supra-gingival plaque on teeth, the keratinized gingiva, the buccal mucosa, the throat, and saliva.
And surprisingly, even though your tongue touches the roof of your mouth, if you rub a Q-tip on either spot I can tell you with 100 percent certainty which surface you just sampled. The organisms living on your tongue are a very different community from what’s on the roof of your mouth.
Why are they so different?
JMW: From the point of view of a bacterium, it matters what kind of surface you’re living on. The teeth are solid, they’re always there. If you can root yourself onto them, you’re not going to get dislodged unless someone pushes you off with a toothbrush or something. Bacteria such as Corynebacteria precipitate calcium from saliva. It’s thought that they turn into that calculus that your dentist scrapes off your teeth. They grow very slowly, but they thrive by gluing themselves to their surface.
But if you’re on the cheek cells, which shed pretty frequently, you have to bind quickly and grow rapidly. The fundamental limit on the length of time you can be bound to your surface and remain in the mouth is likely to be one of the factors that really structure the bacterial community. Streptococcus do well on the cheeks. They’re the first to show up, they grow quickly and then they move on.
How many microbes are in the mouth?
FD: We don’t really know the number of bacteria in an average mouth. But there are something like 1011 [100 billion] organisms per gram of plaque—so we’re looking at a large number.
What people usually talk about is how many species are in there. The Human Oral Microbiome Project identified a little over 700 different species of bacteria. (There are also fungi and viruses.)
About 400 of the 700 bacterial species are much more common in people than the others. And were you to take a swab of the cheek and sequence, sequence, sequence until you saw everything you could, there’d probably be somewhere between 200 and 300 organisms. They would be distributed almost on a logarithmic scale, with the most common organism making up 10 percent of the population, the second organism 5 percent, the third just 2 percent and very rapidly, by the time you get to the 50th, you’re down to 0.1 percent of the population. There’s this long tail.
Since we eat and drink, we take in all of the other microorganisms from the planet. A splash of sea water, some dirt on your spinach. Eventually, if you sampled enough people, enough times, every microorganism on the planet could show up in somebody’s mouth.
GB: You could say the mouth is almost like an open sewer but that may take it too far. Only some of the organisms really take up residence and live there on a regular basis.
JMW: Dental plaque and the surface of the tongue are among the densest microbial habitats on Earth. Bacteria are pretty much wall to wall in there.
I thought bacteria was what plaque was. There’s other stuff in there?
JMW: The bacteria secrete stuff.
GB: It’s called the “extracellular matrix,” or “extra-polymeric substance” …
JMW: Or slime! Plaque is a biofilm—bacteria adhered to a surface, embedded in a matrix of their own making. And biofilms are cool. Bacteria behave differently in a biofilm. There are parts of their metabolism they only turn on in a biofilm, and they tend to be more resistant to antibiotics and changes in the environment. A lot of the material in dental plaque biofilm is DNA, which is interesting. Do the bacteria die and spread their DNA all over the place?
What led you to start making fluorescent images of the colonies formed by the bacteria?
GB: We had a gap in our understanding of microbiome organization. DNA sequencing gave us a catalogue of bacterial genomes, but it had a big limitation: You have to grind up your sample to get the DNA, and in the process you lose all the spatial information—who is next to whom.
This had been a missing piece of the jigsaw puzzle of understanding microbiomes. We realized we could develop imaging tools to see the members, in their habitat, in as close to their normal arrangement as possible.
Why is that so important?
JMW: If you can see who a bacterium is next to, then you’re more likely to understand whom they’re interacting with. That’s important because if we want to recognize what an unhealthy microbiome is—and maybe figure out how to shift it into a healthier state—we need to understand how the bacteria work together. If there’s a particular microbe you want to get rid of, you need to know what else is there next to it, helping it grow or ready to take its place.
GB: Consider a watch (before they became digital). You have so many springs; you have so many wheels; you have a glass surface; you have a metal back; you have a couple jewels. But how does the watch work? Having the parts list is not sufficient. You have to know how the parts fit together, and how one affects another. With DNA sequencing we’re given the parts list, but we’re not told how they work together. If you want to understand the function you have to know the structure.
What do your images show?
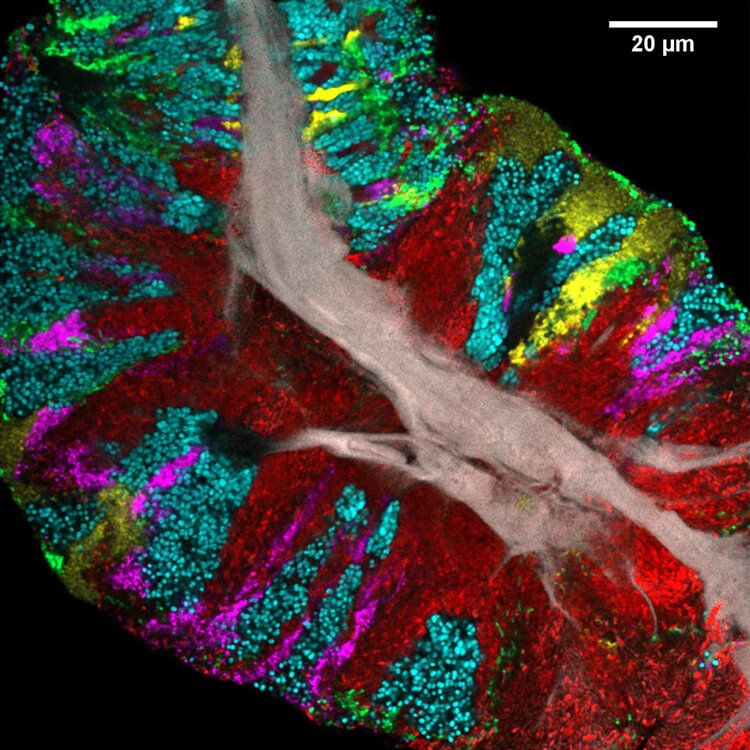
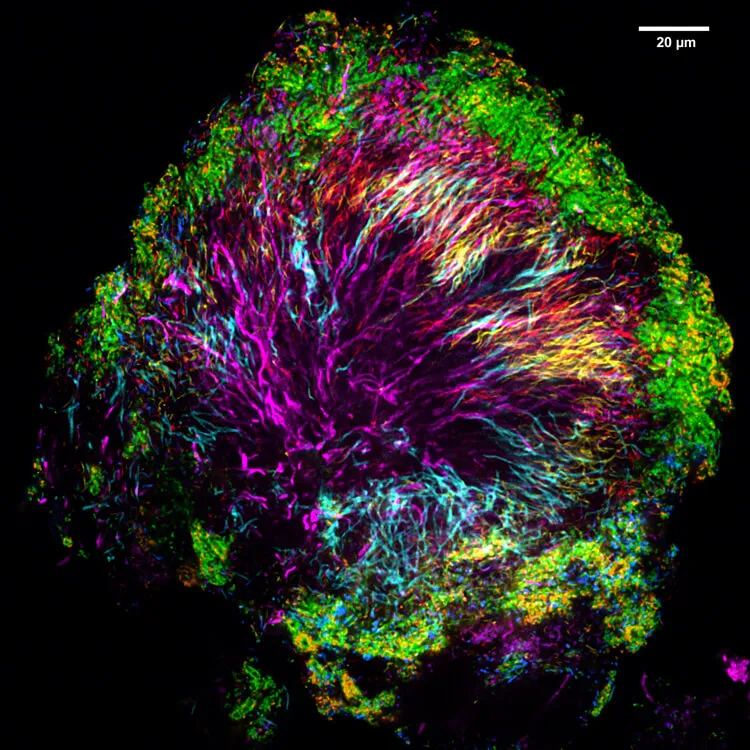
JMW: Vast differences between the structures and make-ups of different parts of this oral ecosystem. For instance, if you look at images of dental plaque and of a microbial community on the tongue, they’re just completely different.
The plaque is characterized by a shape of bacterial community we call a hedgehog, organized around Corynebacteria (in the image, these are the magenta-purple filaments that radiate out from the center.) We think the Corynebacteria are the foundation of community, acting like the coral in the reef or the oak tree in the forest—creating the habitat that other organisms then inhabit at characteristic positions. The ring of bacteria we’ve colored green that you see around the outside of the structure are Streptococcus, and they stay in the aerobic zone, exposed to oxygen. They appear to be creating a low-oxygen zone in the interior that’s been occupied by different bacteria.
But if you look at a microbial community scraped from the surface of the tongue, you see a gray core—dead human epithelial cells—with other bacteria forming these very dense communities growing outwards and expanding together.
FD: With the bacteria in the plaque, it’s almost like you take your fingers and intertwine them—almost every neighboring cell is a different species. But on the tongue, you have these big chunks of blue or red or another color, with cells favoring proximity to cells of the same species.
And this overarching structure has a function in the mouth, presumably?
JMW: Right. Looking at the spatial organization of bacteria in the mouth tells you which microbes are directly attached to the host, and which have the most opportunity to interact with it and its metabolism.
We know that some bacteria in the mouth participate in our nitrate metabolism—how we take in nutrients from food, which can actually modulate blood pressure. If you consume a diet that is rich in nitrate, rich in green leafy vegetables, it will lower your blood pressure a little bit, but not if you use antiseptic mouthwash. In my opinion that might be one reason—and this may be going out on a limb—why we, as the host, allow the bacteria to grow to such density. We have a reason to let them do that.
Researchers are trying to learn more about the ways microbes are implicated in periodontitis (gum disease) and caries (cavities). A common mouth bacterium known as Fusobacterium nucleatum seems to be involved in colon cancer. It’s famous among oral microbiology people because it binds to everything. If it’s attached to harmless Streptococcus, it can evade the immune system and enter the body through the cheek cells, and it probably gets into the colon just by being swallowed.
GB: Some bacteria provide a service to the host, but some turn against us. If we drink a lot of sugary beverages, bacteria that like the sugar thrive, and produce acid that creates cavities. If these get into our bloodstream, they can cause serious disease, such as heart-valve infections. It’s like a garden. When plants aren’t growing where they should, we call them weeds, even though in other places they’d be just fine.
JMW: When we ask volunteers to give us their dental plaque, we ask them to please not brush their teeth for 24 or 48 hours before we take our samples—and we have to ask them whether they have valvular heart disease. It can be especially hazardous for people with valvular heart disease to let these bacteria build up in their mouths.
So yes. These bacteria can provide a benefit to us, but they can hurt us too … and if we want to fight these pathogens we have to understand structure. A microbe’s behavior depends on where it is. A lot of times research is conducted on a single bacterium, in culture. But that bacterium is going to act differently if it’s next to another bacterium. We need to study both together if we really want to understand what they’re doing in the wild. If we figure out which are next to each other in the various locations of the mouth, we know which ones to put in the petri dish.
Scientists have suggested that different parts of the mouth have different bacterial communities for some time. But people still like to sample saliva to measure bacteria in dental plaque. It’s easy. But saliva is a mixture of bacteria from different sites in the mouth and, it turns out that they are mostly tongue bacteria, not plaque. The notion that there is location-specific structure hasn’t sunk in, which is one reason we wanted to write the article.
Where else can scientists look to better understand microbe communities in the human body?
GB: Most people are already looking at the gut. But probably every part of the body will have a distinctive microbiome—the ear, the nose, the belly button, the vaginal tract—and interesting structures.
JMW: I've been trying to flip this around the other way, looking at where else in the world—beyond the human body—you can find interesting spatial structures like those in the human mouth.
It’s taken me full circle back to marine organisms. Kelp and other macroalgae are similar to the mouth, in a way. There’s a fixed surface that’s nutrient-rich, and immersed in flowing water, and that promotes structure in the community.
Kelp is an ecosystem engineer. It is important as habitat for fish and other organisms and for regulating the transfer of nitrogen and carbon. We’re interested in the degree to which the bacteria might be needed for this. How much does the kelp act by itself, and how much does it require microbes to do its work? Analyzing what’s going on in the human mouth might get us closer to an answer.


