The Rise of the Multi-Talented Adult Stem Cell
A new type of cell could lead to dramatic cures—and avoid ethical controversy
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Tabula-Rasa-illustration-631.jpg)
You started out as a single cell. It divided, and so did its daughters and granddaughters, eventually producing trillions of cells with specific identities—red blood cells, neurons, heart muscle cells that beat, beat, beat. For as long as biologists have studied this maturation process, they’ve believed that cells in adult tissue can’t readily take on a whole new identity. But researchers are challenging this idea with the startling discovery of adult cells that retain their flexibility—a possible boon for treating devastating diseases.
The new work is the latest in a series of breakthroughs involving what are called pluripotent (for “many potentials”) stem cells, which give rise to any specialized cell type. In 1998, researchers first isolated human embryonic stem cells, but research on them has been hampered because it requires harvesting cells from discarded human embryos. In 2006, Shinya Yamanaka, of Kyoto University, avoided that ethical controversy when he discovered that adult skin cells can be removed from the body and genetically reprogrammed to revert to a pluripotent state. The work won him last year’s Nobel Prize.
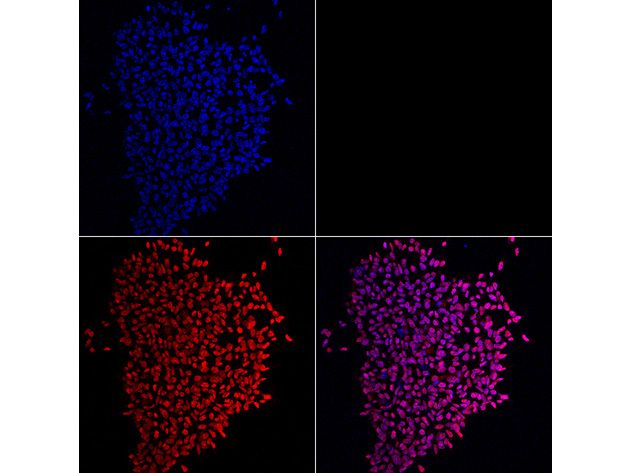
What’s surprising about the new stem cell breakthrough is that researchers don’t have to turn back the cellular clock. Molecular pathologist Thea Tlsty and colleagues at the University of California, San Francisco, had been studying wound-healing cells in the breast, known to divide furiously in response to injury, when they hit upon a small subset carrying surface molecules similar to those on pluripotent stem cells. About 1 in every 10,000 breast cells appears to belong to a class of stem cells never seen before, now dubbed “endogenous pluripotent somatic” cells.
After putting these cells onto a plastic plate and letting them stew in nutrients and growth factors known to nurture the development of heart muscle cells, Tlsty’s junior colleague Somdutta Roy created heart cells that actually beat in the lab dish. “When she first saw the beating cardiomyocytes, she did a little dance,” Tlsty says. “Then she called everybody in the lab over to look at them.” With other nutrient blends, the team brought neurons, bone, fat and blood vessels to life.
If other researchers can replicate the findings, such cells might be used in stem cell therapy, says Deepak Srivastava, of the Gladstone Institute of Cardiovascular Disease in San Francisco. Sick or damaged cells—whether pancreatic cells that perish in diabetes or brain neurons ravaged by Parkinson’s disease—might someday be replaced by healthy counterparts generated from a patient’s own stem cells. These replacements might even grow new organs. “What we used to think about the cell, that it’s fixed in its fate, is just not true,” Srivastava says.
But Paul Knoepfler, a stem cell biologist at the University of California, Davis, says he is somewhat skeptical of the new finding on evolutionary grounds. “Why would nature give an adult tissue these kinds of cells?” he asks.
Further testing will reveal whether nature did or didn’t bestow us with this bounty. But given our humble beginnings, perhaps a single cell’s ability to take on a brand-new identity shouldn’t be terribly surprising.