Scientists Pumped Ovarian Tissue Full of Sugar and Microwaved It. Here’s Why
Though only tried in cat tissues so far, the technique could someday aid fertility preservation, wildlife conservation and more
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/ef/4d/ef4d0f0e-f972-46d8-8e8b-a7a0b9bd0068/20191202_002pc.jpg)
Brine shrimp have a neat trick up their sleeves. When dried out, these teeny crustaceans will fill their cells with a sugar called trehalose that suspends molecules within a glassy matrix, preserving them like fossils in amber. Here’s what makes the situation even sweeter: Plop the brine shrimp into water, and they’ll spring back to life, good as new.
While that’s swell for brine shrimp, most other animals haven’t had the ability to dry their tissues into suspended animation—until now.
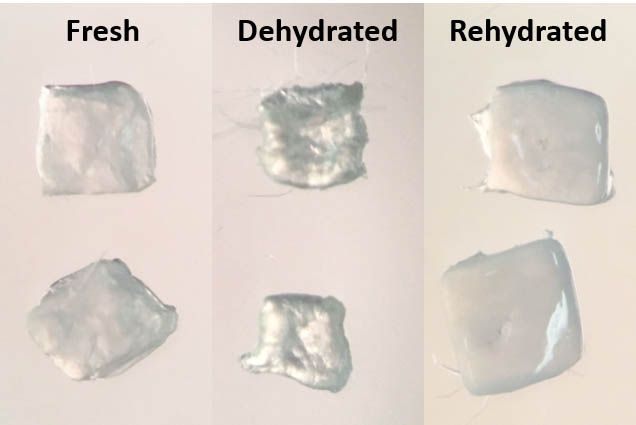
Inspired by creatures like brine shrimp, Smithsonian scientists used trehalose—and a commercial microwave—to dry and preserve living cat ovarian tissue at temperatures above freezing, they report in a recent study published in PLOS ONE. Though some cells sustain damage from the dehydration process when revived, many survive and appear to retain basic functions, including transcribing their DNA in preparation for protein production.
The study is the first to show that pieces of these delicate organs, which harbor eggs in various stages of development, can be safely stored through the simple act of drying, and may remain viable in the aftermath. While the procedure is still in its early stages and has yet to move into human samples, it could someday give researchers an efficient, cost-effective way to safeguard tissues for fertility preservation, wildlife conservation and organ transplantation.
“This is the first step toward long-term preservation [by drying],” says Yuting (Fanny) Fan, a reproductive endocrinologist at the University of Michigan who wasn’t involved in the study. “There’s a long way to go before this can be used as a routine method to preserve tissue. . . but it’s a very innovative approach.”
Smithsonian Conservation Biology Institute (SCBI) researchers Pei-Chih Lee and Pierre Comizzoli designed the technique, aptly named microwave-assisted dehydration, as a potential alternative to cryopreservation, in which living tissues are cooled and stored at very low temperatures with the help of liquid nitrogen.
Cryopreservation is currently “the gold standard for preserving living biomaterials for the long term,” Comizzoli says. But maintaining this chill requires a lot of expensive equipment, energy and human labor, and excludes under-resourced parts of the world from utilizing the technique and burdening researchers where it’s widely deployed.
At its best, microwave-assisted dehydration could circumvent some of these issues. If dried into a stable state, tissues could be kept on shelves—no refrigerators or hazardous materials necessary—potentially cutting the typical cost of cryopreservation by up to 90 percent, says Comizzoli, who’s been working on preserving reproductive tissues for much of his career. But technology isn’t ready to support room temperature storage just yet.
A few years ago, Comizzoli and his team successfully used microwave-assisted dehydration on cat eggs and sperm—the single-celled units of reproduction. The latest study, performed in complex, multicellular tissues, brings the researchers one step closer to their goal.
The method is pretty straightforward, Lee explains. After first slicing ovarian tissue into small chunks, the researchers treated the samples with a chemical that makes cells porous and flooded them with a solution of trehalose. Once the cells were full of sugar, they began the slow process of drying the tissues out in a commercial microwave set to 20 percent power.
Intermittently pulsing the tissues with microwaves steadily zapped them of their water without skyrocketing their temperature, Lee explains. As the cells dried out, the sturdy bits of trehalose cushioned them, preventing collapse.
“Everything is stabilized in a glass,” Comizzoli says. “You suspend interactions between the molecules and their biophysical properties. . . that’s how you are able to suspend life.”
The tissues were then stored for about a day at 39 degrees Fahrenheit—the temperature of a standard refrigerator—before being rehydrated in water. (Eventually, Comizzoli would like to get the tissues stable enough to last without refrigeration.)
Lee and her colleagues tinkered with every step, including laboriously trying out different stints in the microwave. Almost across the board, the researchers were able to revitalize cells that had been dried for up to 30 minutes. Though there were some casualties, most plumped back up to their normal shape in water, and seemed to have their DNA intact.

Looking fine, however, isn’t the same as working fine, and it’s still unclear just how functional the rehydrated cells are. The longer the tissues were microwaved, the more basic cellular processes like DNA transcription—the first step in protein production—were compromised. And when ovarian tissue was dried for more than ten minutes, only a small fraction of the follicles, which contain immature eggs, survived.
Comizzoli stresses that these experiments primarily demonstrate proof-of-concept—to show that a more fine-tuned procedure would even be possible. “Now that we have that, we are [trying to] improve everything,” he says.
The team is already in the midst of experiments to optimize their protocol by simultaneously maximizing preservation and minimizing cell damage. Once those parameters are a little clearer, he says, they’ll start testing how well the reanimated tissues perform at their intended function: reproduction.
What the team is trying to accomplish is “a difficult feat,” says Monica Laronda, a fertility preservation expert at Northwestern University who wasn’t involved in the study. Some of the most important experiments are those yet to come, including assessing the health of tissues after much longer periods of storage. Eventually, the team will want to see if the tissues keep bouncing back in the days and weeks after resuscitation—or even survive a transplant back into a living body.
If the team’s success continues, the researchers ultimately plan to expand their technique into other mammalian species. At SCBI, Comizzoli and his colleagues have long been engaged in preserving and restoring the world’s biodiversity. Part of their mission is to oversee the breeding of endangered species and their subsequent reintroduction in the wild—something that could get a huge boost from a freezer-free method for storing and transporting reproductive tissues.
Shifting into human medicine is much further out of reach, but certainly appealing. One group that could benefit immensely from the technique includes female cancer patients who need to undergo chemotherapy, a harsh procedure that often destroys fragile reproductive tissues. To safeguard their ovaries—and the eggs within—some women turn to cryopreservation to freeze the tissue while they finish treatment, Fan says. Microwave-assisted dehydration could someday offer these patients a cheaper, more convenient option.
There’s also no reason the technique couldn’t be used in other tissue types, Comizzoli says.
Cooling techniques have already made their way into organ transplantation research, and the same could certainly be attempted with dehydration. If that pans out, then it might be a good thing that the first tests were in reproductive tissues, he says, because “they’re the most complicated ones.”
Cryopreservation is a big industry, but it doesn’t have to have a monopoly over tissue preservation. “It’s inconvenient,” Fan says. “That’s why it’s critical to study other methods.”
There’s a long way to go before microwave-assisted dehydration becomes a serious contender, but with studies like these, she says, there’s already some hope.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/10172852_10152012979290896_320129237_n.jpg)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/10172852_10152012979290896_320129237_n.jpg)